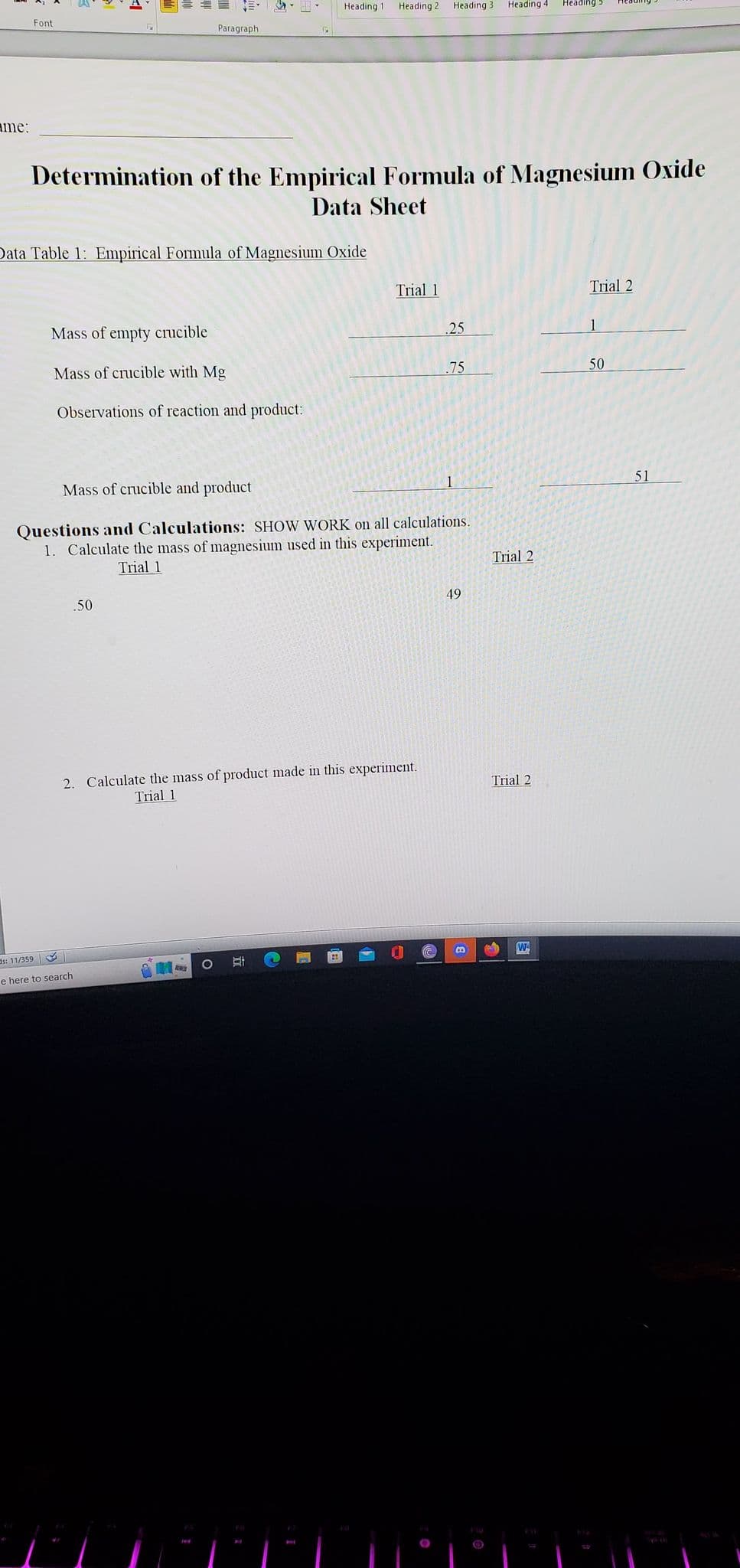
Determination of the Empirical Formula of Magnesium Oxide Data Sheet ata Table 1: Empirical Formula of Magnesium Oxide Mass of empty crucible Mass of crucible with Mg Observations of reaction and product: Trial 1 .50 25 2. Calculate the mass of product made in this experiment. Trial 1 75 Mass of crucible and product Questions and Calculations: SHOW WORK on all calculations. 1. Calculate the mass of magnesium used in this experiment. Trial 1 1 49 Trial 2 Trial 2 Trial 2 50 51
Determination of the Empirical Formula of Magnesium Oxide Data Sheet ata Table 1: Empirical Formula of Magnesium Oxide Mass of empty crucible Mass of crucible with Mg Observations of reaction and product: Trial 1 .50 25 2. Calculate the mass of product made in this experiment. Trial 1 75 Mass of crucible and product Questions and Calculations: SHOW WORK on all calculations. 1. Calculate the mass of magnesium used in this experiment. Trial 1 1 49 Trial 2 Trial 2 Trial 2 50 51
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.13QAP
Related questions
Question
Solve for #2 if #1 is correct.
Transcribed Image Text:ame:
Font
12
Paragraph
Data Table 1: Empirical Formula of Magnesium Oxide
Mass of empty crucible
Mass of crucible with Mg
Observations of reaction and product:
.50
s: 11/359
e here to search
Heading 11
Determination of the Empirical Formula of Magnesium Oxide
Data Sheet
Heading 2
O St
Trial 1
2. Calculate the mass of product made in this experiment.
Trial 1
Heading 31 Heading 4
Mass of crucible and product
Questions and Calculations: SHOW WORK on all calculations.
1. Calculate the mass of magnesium used in this experiment.
Trial 1
.25
.75
49
B
Trial 2
Trial 2
Heading
W
Trial 2
50
51
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

