Determine how many moles of NaOH are required to neutralize a solution containing 1.356 g of KHC3H20 (KHP) dissolved in 75 ml of distilled water. Keep in mind: 1 KHC,H,0, (aa) + 1 NAOH (co) + 1 H,0 (1) + 1 NAKC,H,0, (aq) Molar Mass KHP: 204.22 g/mol
Determine how many moles of NaOH are required to neutralize a solution containing 1.356 g of KHC3H20 (KHP) dissolved in 75 ml of distilled water. Keep in mind: 1 KHC,H,0, (aa) + 1 NAOH (co) + 1 H,0 (1) + 1 NAKC,H,0, (aq) Molar Mass KHP: 204.22 g/mol
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.31E
Related questions
Question
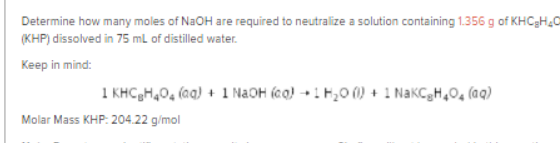
Transcribed Image Text:Determine how many moles of N2OH are required to neutralize a solution containing 1.356 g of KHC3H20
(KHP) dissolved in 75 mL of distilled water.
Keep in mind:
1 KHCgH,0, (aq) + 1 N2OH (cq) + 1 H,0 (0) + 1 NakCgH,0, (aq)
Molar Mass KHP: 204.22 g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning