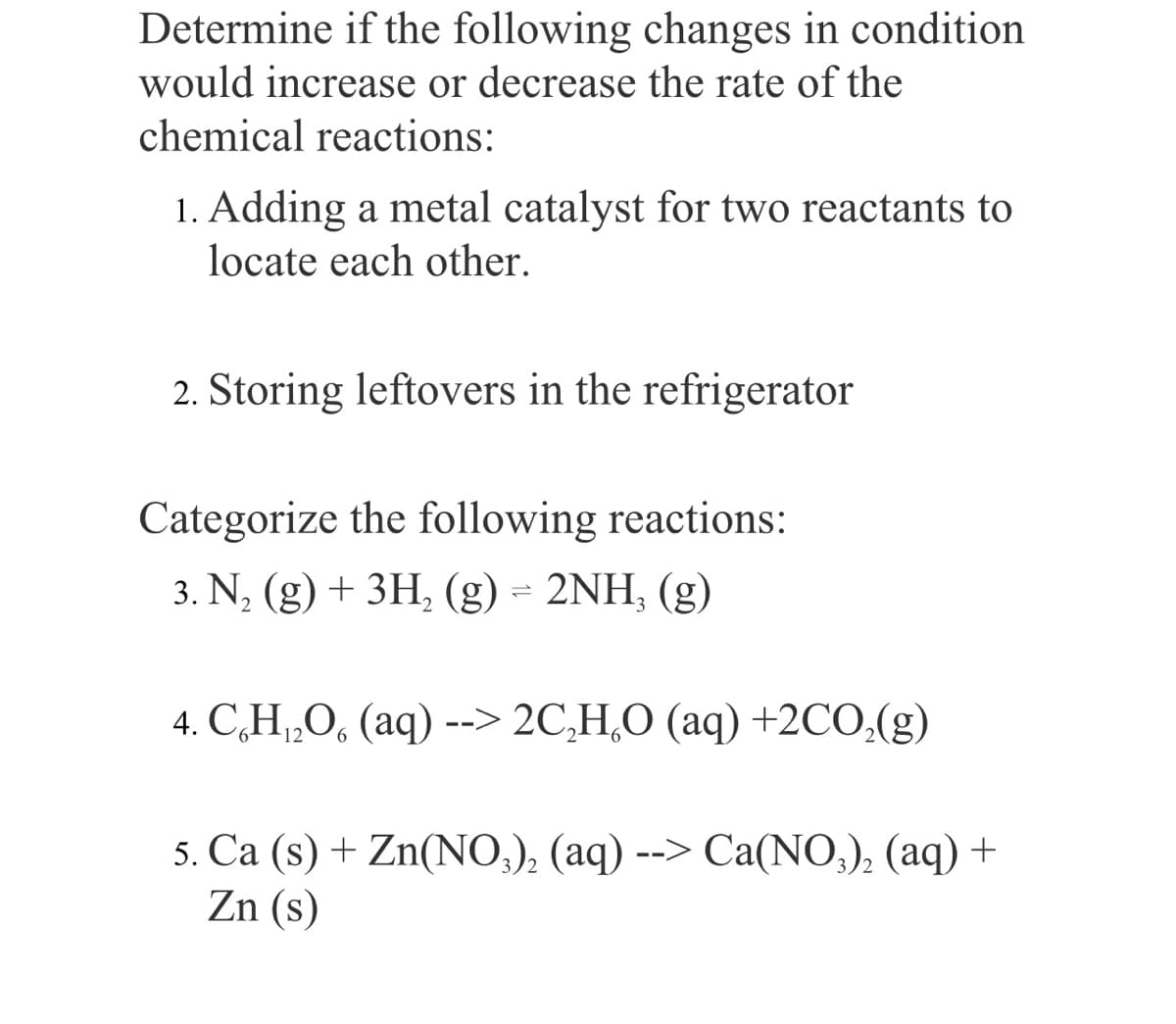
Determine if the following changes in condition would increase or decrease the rate of the chemical reactions: 1. Adding a metal catalyst for two reactants to locate each other. 2. Storing leftovers in the refrigerator
Q: reaction will occur, give the product(s) otherwise write no reaction. Also indicate if the reaction…
A: The answer is as follows:
Q: Which of the following is true of a catalyst for a reaction A It decreases the equilibrium…
A: Catalyst :- A substance which when added to a reaction mixture increases the speed or rate of…
Q: How would you increase the rate of a reaction containing solid reactant without adding more…
A: When we use use a solid Reactants for particular Reaction, then rate of reaction can be increased by…
Q: Explain why the rate of reaction increases with increase in temperature.
A: Increasing of temperature occurs due to provision of heat energy to a system. When that heat energy…
Q: 1. It is defined as the detailed description, step by step, of the same, which means knowing all the…
A: 1. It is defined as the detailed description, step by step, of the same, which means knowing all the…
Q: A burning splint will burn more vigorously in pure oxygen than in air because A) oxygen is a…
A: Solution : A burning splint will burn more vigorously in pure oxygen than in air because : –…
Q: Consider the energy diagram for the four processes and answer the following questions. A B D…
A: We are given with energy diagrams for four processes with different co-ordinates for reactants and…
Q: Why does decreasing concentration decrease the rate of a chemical reaction?
A: The chemical reaction rate is the speed with which the reaction moves ahead. Chemical reactions are…
Q: All of the following effect the rate of a chemical reaction except for which one? increasing the…
A: In this question, we want to identify which one doesn't effect the rate of the chemical reaction.…
Q: Decreasing the temperature typically causes the rate of a chemical reaction to slow down, which of…
A: Collision theory of reaction rate: According to the collision theory of reaction rate, the randomly…
Q: If a reaction will occur, give the product(s) otherwise write no reaction. Also indicate if the…
A:
Q: When a cube of manganese oxide was added to a solution of hydrogen peroxide, water and oxygen were…
A: The rate of the reaction depends upon many factors like temperature concentration pressure etc,…
Q: Classify each of these reactions.
A: Since you have posted a question with sub-parts , we are entitled to answer the first three…
Q: Which of the following changes below speeds up the rate of reaction? Decrease the surface area of…
A: Increasing reactant concentration would speed up the rate of reaction
Q: 60. In this diagram, AH represents Products Reactants Reaction Progress (A) heat absorbed. (B) heat…
A: There are basically two types of reactions - exothermic and endothermic reactions. Endothermic…
Q: If two reactants collided with energy but no reaction occurred, what would be the most likely…
A: There are three important parts to collision theory, that reacting substances must collide, that…
Q: A. If a reaction will occur, give the product(s) otherwise write no reaction. Also indicate if the…
A:
Q: State the two observations that make in the gas jar, write for the equation that will occur, and…
A: 2Na(s) + Cl2(g) ——> 2NaCl(s) Sodium chloride is used to treat or prevent sodium loss caused by…
Q: Decreasing the concentrations of the reacting particles decreases the chance of collision.
A: The factors affecting the rate of the reaction are:
Q: Which of these will slow down a chemical reaction Adding a catalyst Increasing the temperature…
A: Option 3 should be the correct answer Decreasing the concentration of the reactants Because we know…
Q: II.Match each item with the correct statement below. a. Le Chatelier's Principle d. substrate…
A: Le Chateliers principle states that when a stress is applied to a system under equilibrium, the…
Q: There is a chemistry problem. The final answer to this problem is CHEMICAL REACTION. Think of a…
A: The purpose is to ask questions regarding chemical changes happening.
Q: a forward reaction is exothermic and the temperature is increased A. the forward reaction will…
A: Reaction is exothermic. It means change is enthalpy is negative. Heat is evolved.
Q: As you decrease the surface area of a reactant, the rate of the reaction increases or decreases?
A: Rate of reaction depends on the area of the reactants.
Q: Explain why increasing the temperature and/or adding a catalyst will increase the rate of any…
A: The effect of temperature and catalyst on the rate of reaction is explained below.
Q: Which of the following is not a way to stress a chemical system? A. Pressure B. Temperature C.…
A: The state at which forword reaction is equal to back word reaction is said to be " equilibrium…
Q: the factors that affect the rate of a chemical reaction and how they affect?
A: Given: To give the factors that affect the rate of a chemical reaction.
Q: What would happened to the rate of the reaction by decreasing the number of moles of reacting…
A: Here the mentioned three factor greatly influenced the rate of reaction. The concentration,…
Q: Which
A: Structure Y act as a Nucleophile and it will attack on alpha carbon of Structure Z by removing…
Q: Using Le Châtelier's principle, determine how the process is affected after each of the following…
A: According to le-chatelier principle, a change in one of the variables that describe an equilibrium…
Q: 8. Consider the energy diagram for the four processes and answer the following questions. B…
A: a) On comparing the energy diagram of A and D, process D will occur rapidly.
Q: reaction will occur, give the product(s) otherwise write no reaction. Also indicate if the reaction…
A: The answer is as follows:
Q: reaction will occur, give the product(s) otherwise write no reaction. Also indicate if the reaction…
A:
Q: What happens to the reaction rate if the energy of the product is lowered?
A: It is given that the rate of the reaction needs to be determined when the energy of the product…
Q: For any chemical reaction at dynamic equilibrium , what is the rate of the forward reaction?
A:
Q: If heat is given to an endothermic reaction, the reaction will ________ not shift at all…
A: Since the reaction is endothermic, it will proceed in forward direction with increase in temperature…
Q: Which of the following will decrease the rate of a reaction? A) Removing a catalyst from the…
A: Rate of a Reaction depends on various parameters like temperature, concentration of the reactants,…
Q: How does an increase in temperature affect the rate of a reaction? Explain the two factors involved.
A: The decrease in the concentration of the reactant or increase in the concentration of the products…
Q: How do you label an energy diagram of a chemical reaction? What is a catalyst and how does it…
A: The progress of a reaction can be represented by the reaction coordinate diagram. In a reaction…
Q: PREDICT THE REACTANT OF THE REACTION GIVEN:
A:
Q: Select one: a. The catalyst takes a part in the reaction, but it is not consumed during the reaction…
A: Which one of the following is correct
Q: Which of the following statements about a catalyst is true? O Catalysts increase the rate of the…
A:
Q: What may happen to the reaction rate when you add catalyst on it?
A: catalyst increases the rate of reaction but it self doesn't involve in any permanent chemical…
Q: Consider the attached energy diagram for the conversion of A → G. Question: Which points on the…
A: The energy diagram implies that A is the starting material and G is the final product. So, they…
Q: Use the energy diagram for the reaction A → D to answer the questions. How many transition states…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: describe 3 ways to increase the rate of a chemical reaction.
A: Rate or speed of the reaction can be increased in ways as discussed in the following step.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- A 51.0-LL reactor at 1600 KK is charged with 50.0 gg of NiO(s)NiO(s) and 1.40 atmatm of CO(g)CO(g). After equilibrium is reached, what is the partial pressure of CO2(g)CO2(g) in the reactor?Calculate the AGrek value for the following reaction at 298K. CO (g) + 02 (g) - CO2 (g) Assuming that the AH°reak value is constant in the operating temperature range, calculate the AG value at 500K.Draw a dashed line to show the effect of adding a catalyst to the system which decreases EA by 20 kJ. Name 4 things that speed up a reaction:
- In process contact, the material produces phosphoric acid. OneThe reactions in the process are the conversion of the SO2 gas to SO3 gas according to the following reaction:2SO2 (g) + O2 (g) ⇄ 2SO3 (g) ΔH ° = -192 kJ · mol^-1As part of the study of this process, a tank was filled with a mixture of the three gases in quantities that create equilibriumWeight, and add catalyst. The initial temperature in the tank was 450 ° C. The tank has a fixed and insulated volumeFrom the environment. Below is a graph depicting the changes in the concentration of reaction components over time. 1. What response components are represented by the letters X and Y? 2. What change occurred in the response in the tenth minute? 3. Explain the changes that take place in the system between the 10th minute and the 20th minute. 4. Explain the changes that will occur in the system under the influence of heating. 5. What combination of pressure conditions (high / low) and temp.( High / low) will lead to the…The following questions refer to the following reaction at constant 25°C and 1 atm.2Fe(s) + (3/2)O2(g) + 3H2O(l) 2Fe(OH)3(s) H = –789 kJ/molSubstance S° (J/mol K)Fe(OH)3(s) 107Fe(s) 27O2(g) 205H2O(l) 70a. Determine Ssurr for the reaction (in kJ/mol K)b. Determine Suniv for the reaction (in kJ/mol K)2.65c. What is the G for this reaction?d. Calculate the equilibrium constant10. What is ∆G for the decomposition of CaCO3 at 298K and a partial pressure of CO2 of 4*10-4 bar? ∆Gf0 for CaCO3(s), CaO(s) and CO2(g) are -1129 kJ/mol, -604 kJ/mol and -394 kJ/mol, respectively. CaCO3(s) → CaO(s) + CO2(g) A. 0 B. -131 kJ/mol C. 112 kJ/mol D. 131 kJ/mol E. 150 kJ/mol (Correct answer is C, I'm just looking for an explanation!).
- Anda how about the standart potencial? E = Eº - 0,0591/n log kpsThe value of delta G at 281.0oC for the formation of phosphorous trichloride from its constituent elements, P2 (g) + 3CI2 (g) ---> 2PCI3 (g) is _____ kJ/mol. At 25.0oC for this reaction delta H is -720.5kJ/mol, delta G is -642.9 kJ/mol, and delta S is -263.7 J/K.consider the following balancing process at 700c 2H2 (g) + 2s2 (g) 《》 2H2 (g) An analysis shows that there are 2.5 moles of H2, 135 × 10 ^ -5 moles of S2 and 8.70 moles of H2S contained in a 12-liter flask calculating Kc and Kp
- At 25oC, for the reaction 2 A(aq) ----> B(aq) + C(aq), the equilibrium constant is 1.79 . If the concentration of B(aq) were 0.311 M and the concentration of C(aq) were 0.477 M, what would be the minimum concentration of A(aq) required (in mol/L) to make this reaction spontaneous under these conditions?At 100 oC, Keq = 1.5E8 for the reaction:CO(g) + Cl2(g) COCl2(g)Using appropriate approximation, calculate the partial pressure of CO at 100 oC at equilibrium in a chamber that initially contains COCl2 at a pressure of 0.317 bar.The following reaction was carried out in a 4.00 LL reaction vessel at 1100 KK: C(s)+H2O(g)⇌CO(g)+H2(g)C(s)+H2O(g)⇌CO(g)+H2(g) If during the course of the reaction, the vessel is found to contain 7.25 molmol of CC, 15.5 molmol of H2OH2O, 3.80 molmol of COCO, and 6.10 molmol of H2H2, what is the reaction quotient QQQ? Enter the reaction quotient numerically.

