Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.21QAP
Related questions
Question
Determine the mass of deposited copper from the data in Table 8b-1. These will serve as the experimental values.
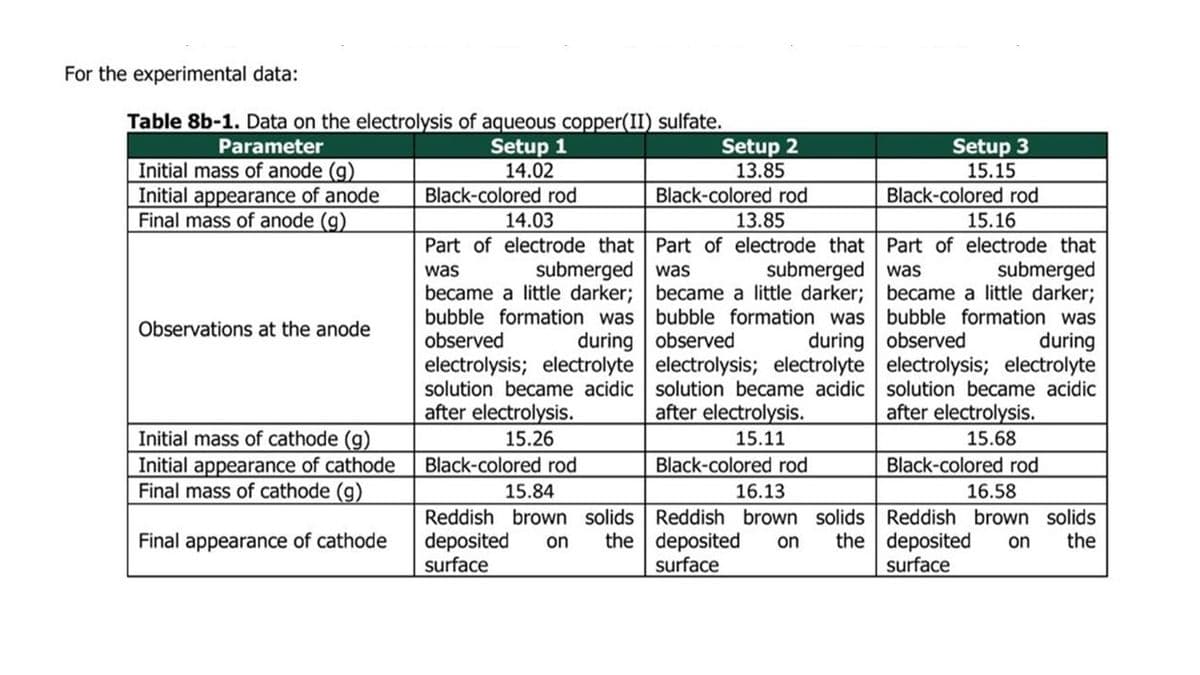
Transcribed Image Text:For the experimental data:
Table 8b-1. Data on the electrolysis of aqueous copper(II) sulfate.
Parameter
Setup 1
14.02
Black-colored rod
14.03
Part of electrode that
was
submerged
became a little darker;
bubble formation was
observed
during
electrolysis; electrolyte
solution became acidic
after electrolysis.
15.26
Black-colored rod
15.84
15.16
Part of electrode that
was
submerged
became a little darker;
bubble formation was
observed
during
electrolysis; electrolyte
solution became acidic
after electrolysis.
15.68
Black-colored rod
16.13
16.58
Reddish brown solids
Reddish brown solids
Reddish brown solids
deposited on the deposited on the deposited on the
surface
surface
surface
Initial mass of anode (g)
Initial appearance of anode
Final mass of anode (g)
Observations at the anode
Initial mass of cathode (g)
Initial appearance of cathode
Final mass of cathode (g)
Final appearance of cathode
Setup 2
13.85
Black-colored rod
13.85
Part of electrode that
was submerged
became a little darker;
bubble formation was
observed during
electrolysis; electrolyte
solution became acidic
after electrolysis.
15.11
Black-colored rod
Setup 3
15.15
Black-colored rod
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
