Determine the most likely structure of a compound, with the molecular formula C9H12, which gave a ¹H NMR spectrum consisting of: a doublet at d 1.25 Oll I OV OIV ||| a septet at d 2.90 and a multiplet at d 7.25 II III IV
Determine the most likely structure of a compound, with the molecular formula C9H12, which gave a ¹H NMR spectrum consisting of: a doublet at d 1.25 Oll I OV OIV ||| a septet at d 2.90 and a multiplet at d 7.25 II III IV
Chapter21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions
Section21.SE: Something Extra
Problem 80AP: Draw the structure of the compound that produced the spectra below. The infrared spectrum has strong...
Related questions
Question
Determine the most likely structure of a compound with the formula C9H12 if it gave an H NMR spectrum consisting of:
A doublet at d 1.25, a septet at d 2.90 and a multiplex at d 7.25
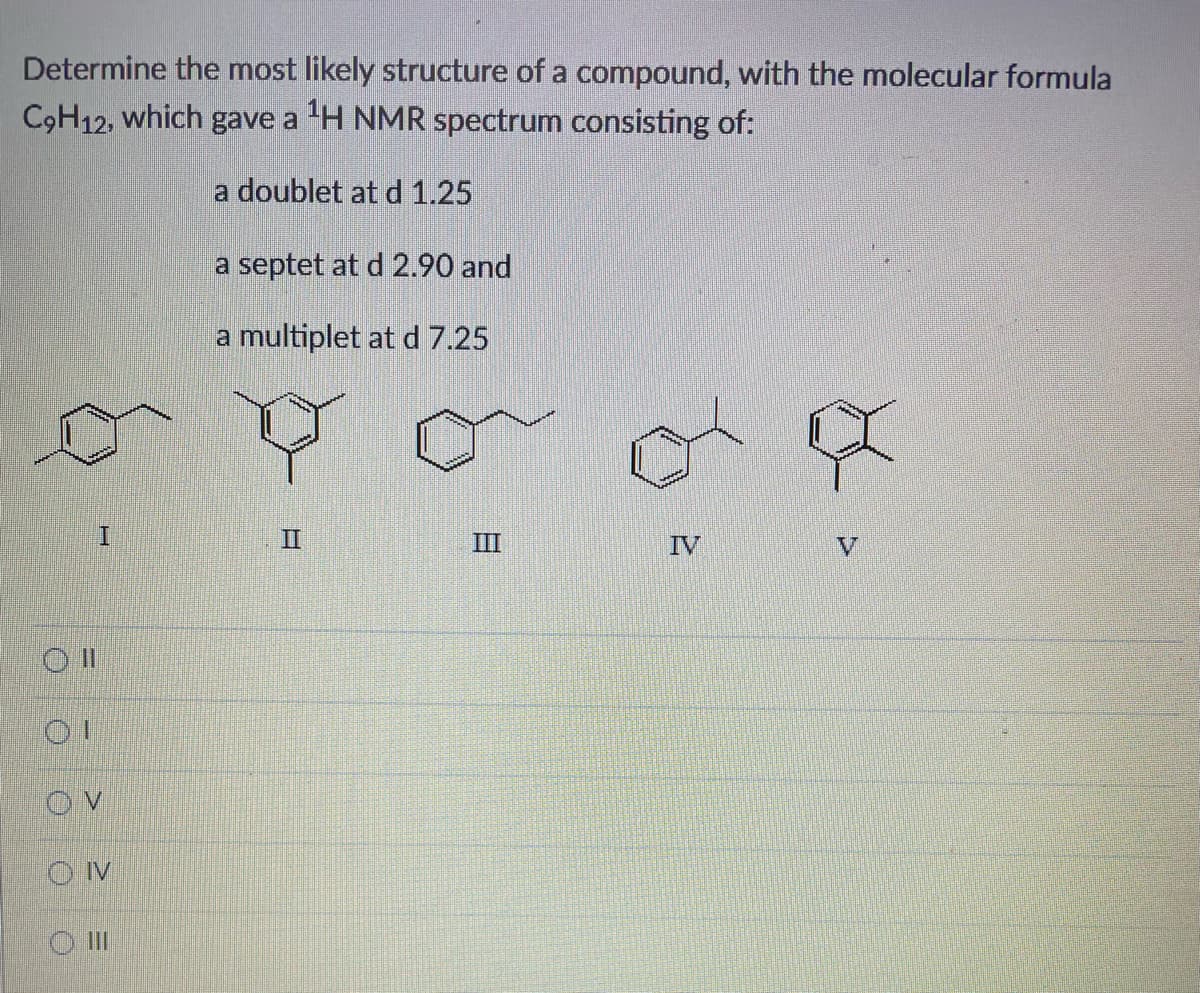
Transcribed Image Text:Determine the most likely structure of a compound, with the molecular formula
C₂H12, which gave a ¹H NMR spectrum consisting of:
a doublet at d 1.25
O
O
I
OV
ON
OIII
a septet at d 2.90 and
a multiplet at d 7.25
IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

