Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.34E
Related questions
Question
Chapter 2 guidelines
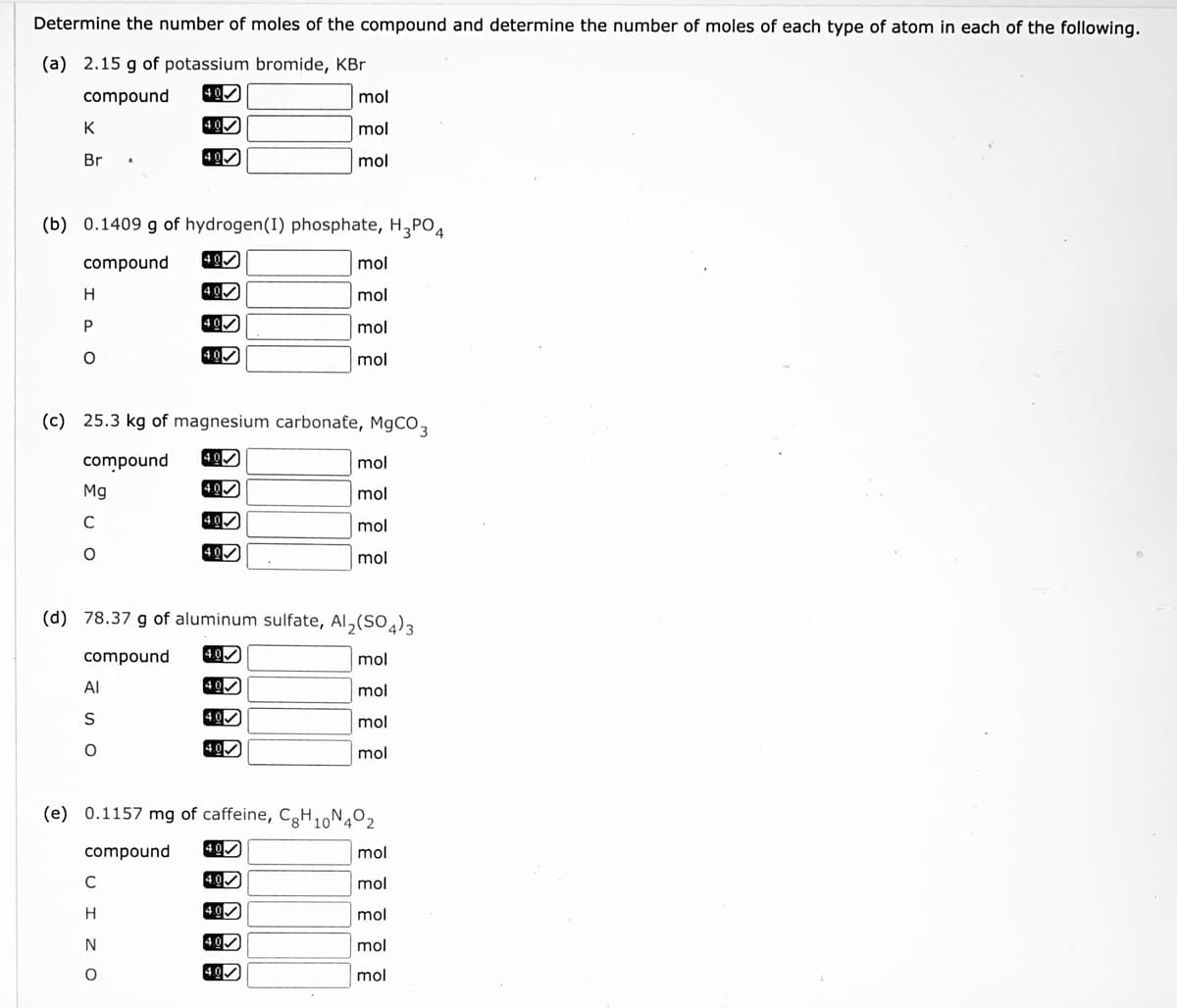
Transcribed Image Text:Determine the number of moles of the compound and determine the number of moles of each type of atom in each of the following.
(a) 2.15 g of potassium bromide, KBr
compound
40
mol
K
mol
Br
40
mol
(b) 0.1409 g of hydrogen(I) phosphate, H,PO4
compound
400
mol
40
mol
P
mol
4.00
mol
(c) 25.3 kg of magnesium carbonate, MgCO3
compound
4.0
mol
Mg
4.0
mol
C
4.0
mol
40
mol
(d) 78.37 g of aluminum sulfate, Al,(SO4)3
compound
4.0
mol
Al
mol
S
mol
mol
(e) 0.1157 mg of caffeine, CgH,N4º2
compound
40
mol
C
4.0
mol
4.0
mol
4.0
mol
4.0
mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

