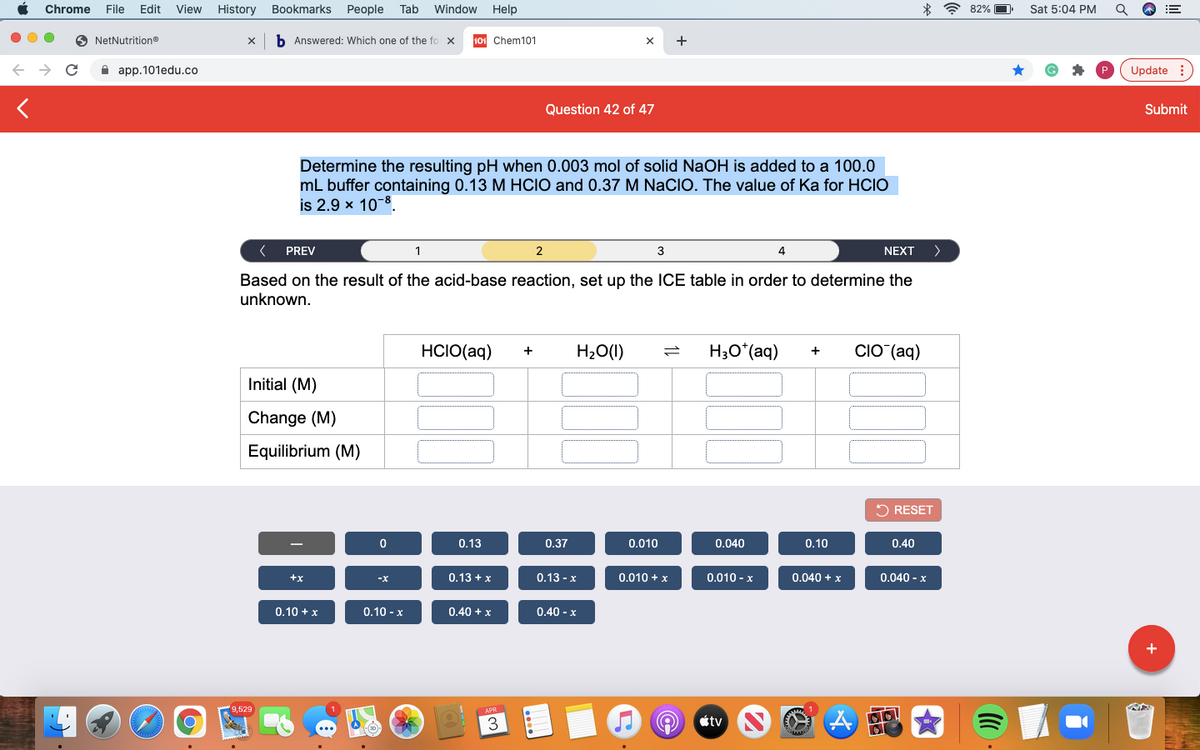
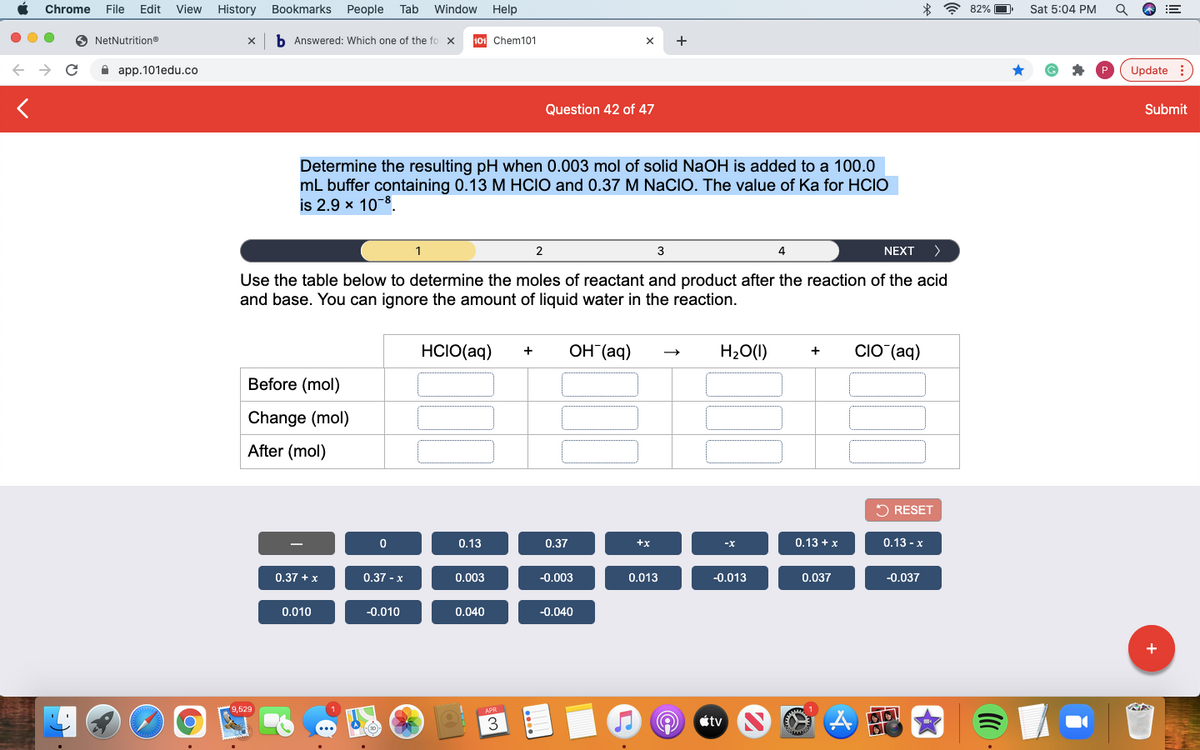
Determine the resulting pH when 0.003 mol of solid NaOH is added to a 100.0 mL buffer containing 0.13 M HClO and 0.37 M NaClO. The value of Ka for HClO is 2.9 × 10⁻⁸
Determine the resulting pH when 0.003 mol of solid NaOH is added to a 100.0 mL buffer containing 0.13 M HClO and 0.37 M NaClO. The value of Ka for HClO is 2.9 × 10⁻⁸
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 118QRT
Related questions
Question
Determine the resulting pH when 0.003 mol of solid NaOH is added to a 100.0 mL buffer containing 0.13 M HClO and 0.37 M NaClO. The value of Ka for HClO is 2.9 × 10⁻⁸
Transcribed Image Text:Chrome
File
Edit
View
History
Bookmarks
People
Tab
Window
Help
* ? 82%
Sat 5:04 PM
NetNutritiono
b Answered: Which one of the fo x
101 Chem101
+
A app.101edu.co
Update :
Question 42 of 47
Submit
Determine the resulting pH when 0.003 mol of solid NaOH is added to a 100.0
mL buffer containing 0.13 M HCIO and 0.37 M NaCIO. The value of Ka for HCIO
is 2.9 x 10-8.
PREV
2
3
4
NEXT
>
Based on the result of the acid-base reaction, set up the ICE table in order to determine the
unknown.
HCIO(aq)
H20(1)
H3O*(aq)
CIO (aq)
+
Initial (M)
Change (M)
Equilibrium (M)
O RESET
0.13
0.37
0.010
0.040
0.10
0.40
+x
0.13 + x
0.13 - x
0.010 + x
0.010 - x
0.040 + x
0.040 - x
-X
0.10 + x
0.10 - x
0.40 + x
0.40 - x
9,529
3
étv
Transcribed Image Text:Chrome
File
Edit
View
History
Bookmarks
People
Tab
Window
Help
* ? 82%
Sat 5:04 PM
O NetNutrition®
b Answered: Which one of the fo x
101 Chem101
+
A app.101edu.co
Update :
Question 42 of 47
Submit
Determine the resulting pH when 0.003 mol of solid NaOH is added to a 100.0
mL buffer containing 0.13 M HCIO and 0.37 M NaCIO. The value of Ka for HCIO
is 2.9 x 10-8.
1
2
3
4
NEXT
>
Use the table below to determine the moles of reactant and product after the reaction of the acid
and base. You can ignore the amount of liquid water in the reaction.
HCIO(aq)
OH (aq)
H20(1)
CIO (aq)
+
+
Before (mol)
Change (mol)
After (mol)
2 RESET
0.13
0.37
+x
0.13 + x
0.13 - x
0.37 +x
0.37 - x
0.003
-0.003
0.013
-0.013
0.037
-0.037
0.010
-0.010
0.040
-0.040
9,529
3
étv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning