Determine the thickness of iron needed to achieve the following shielding goal. Iron density is 7.8 g/cm³. Fe atomic mass is 55.845u Iron mass attenuation coefficient for 1.5 MeV photon is 0.0535 cm²/g. (a) Stop 1.5 MeV alpha particle. (b) Stop 1.5 MeV beta particle. (c) Reduce the 1.5 MeV photon intensity to 1%.
Determine the thickness of iron needed to achieve the following shielding goal. Iron density is 7.8 g/cm³. Fe atomic mass is 55.845u Iron mass attenuation coefficient for 1.5 MeV photon is 0.0535 cm²/g. (a) Stop 1.5 MeV alpha particle. (b) Stop 1.5 MeV beta particle. (c) Reduce the 1.5 MeV photon intensity to 1%.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter32: Radiochemical Methods
Section: Chapter Questions
Problem 32.2QAP
Related questions
Question
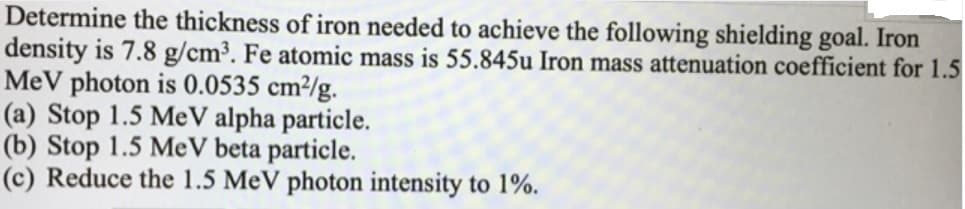
Transcribed Image Text:Determine the thickness of iron needed to achieve the following shielding goal. Iron
density is 7.8 g/cm³. Fe atomic mass is 55.845u Iron mass attenuation coefficient for 1.5
MeV photon is 0.0535 cm²/g.
(a) Stop 1.5 MeV alpha particle.
(b) Stop 1.5 MeV beta particle.
(c) Reduce the 1.5 MeV photon intensity to 1%.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 7 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning