Direction: Relate the properties of liquids to intermolecular forces. Tell whether each property exhibits strong or weak intermolecular forces by putting a check (v) on the appropriate column. Properties of Liquids Intermolecular Forces Strong Weak Surface Tension High Low Viscosity High Low Vapor Pressure High Low Boiling Point High Low Molar Heat of High Vaporization Low MULTIPLE CHOICE. Read and understand the following questions. Circle the letter of the best answer. 1. What happens to the viscosity of syrup when the temperature increases? C. Becomes lower D. Nothing will happen A. Becomes higher B. May become higher or lower 2. Why does an insect float on water? A. Because water has high surface tension. C. The density of the insect is high. B. Because water has low surface tension. D. The density of the insect is low. 3. Which situation shows high vapor pressure? A Open container
Direction: Relate the properties of liquids to intermolecular forces. Tell whether each property exhibits strong or weak intermolecular forces by putting a check (v) on the appropriate column. Properties of Liquids Intermolecular Forces Strong Weak Surface Tension High Low Viscosity High Low Vapor Pressure High Low Boiling Point High Low Molar Heat of High Vaporization Low MULTIPLE CHOICE. Read and understand the following questions. Circle the letter of the best answer. 1. What happens to the viscosity of syrup when the temperature increases? C. Becomes lower D. Nothing will happen A. Becomes higher B. May become higher or lower 2. Why does an insect float on water? A. Because water has high surface tension. C. The density of the insect is high. B. Because water has low surface tension. D. The density of the insect is low. 3. Which situation shows high vapor pressure? A Open container
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 1RQ: What are intermolecular forces? How do they differ from intramolecular forces? What are...
Related questions
Question
Topic: Properties of Liquids
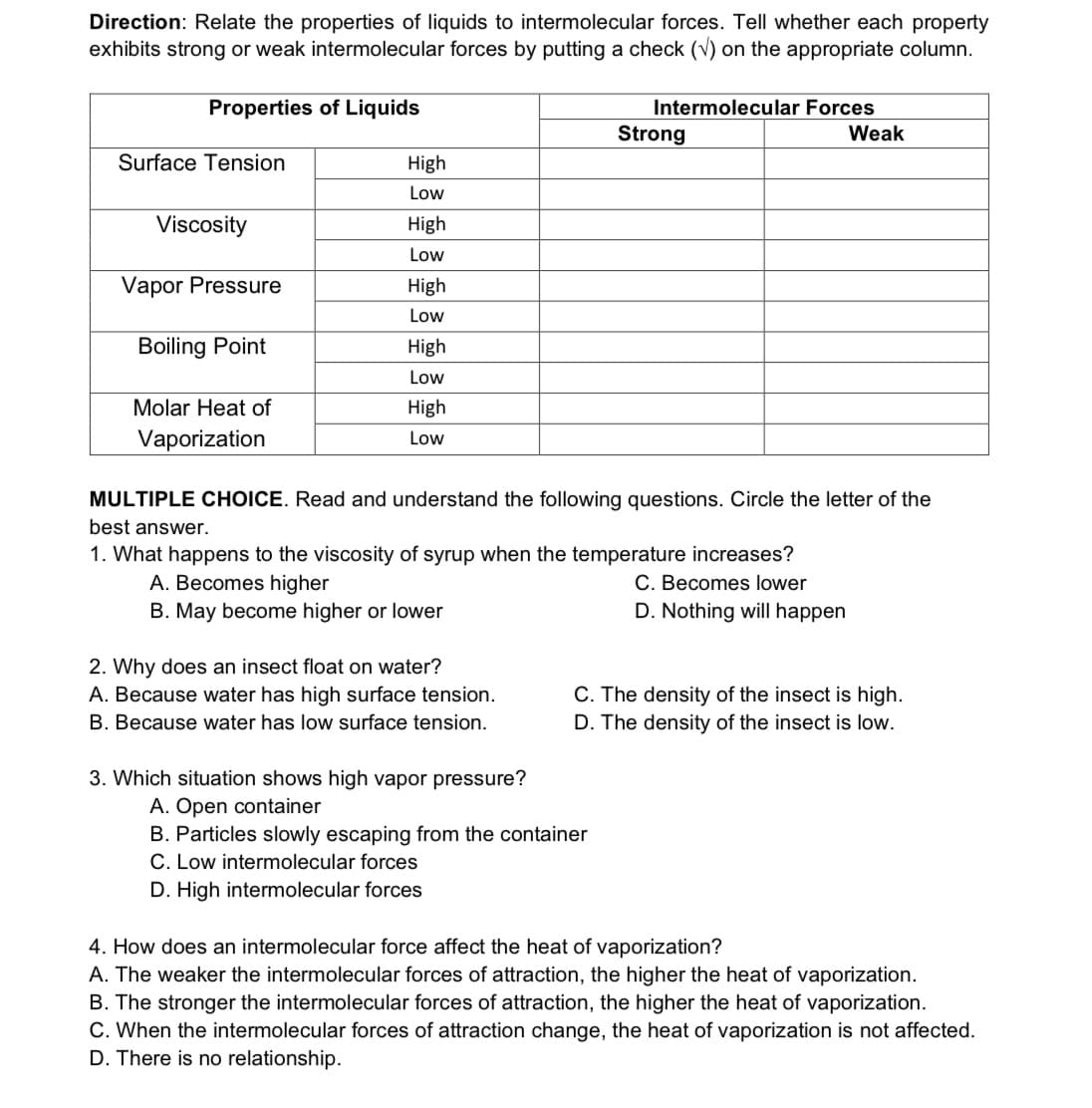
Transcribed Image Text:Direction: Relate the properties of liquids to intermolecular forces. Tell whether each property
exhibits strong or weak intermolecular forces by putting a check (v) on the appropriate column.
Properties of Liquids
Intermolecular Forces
Strong
Weak
Surface Tension
High
Low
Viscosity
High
Low
Vapor Pressure
High
Low
Boiling Point
High
Low
Molar Heat of
High
Vaporization
Low
MULTIPLE CHOICE. Read and understand the following questions. Circle the letter of the
best answer.
1. What happens to the viscosity of syrup when the temperature increases?
C. Becomes lower
D. Nothing will happen
A. Becomes higher
B. May become higher or lower
2. Why does an insect float on water?
A. Because water has high surface tension.
C. The density of the insect is high.
B. Because water has low surface tension.
D. The density of the insect is low.
3. Which situation shows high vapor pressure?
A. Open container
B. Particles slowly escaping from the container
C. Low intermolecular forces
D. High intermolecular forces
4. How does an intermolecular force affect the heat of vaporization?
A. The weaker the intermolecular forces of attraction, the higher the heat of vaporization.
B. The stronger the intermolecular forces of attraction, the higher the heat of vaporization.
C. When the intermolecular forces of attraction change, the heat of vaporization is not affected.
D. There is no relationship.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning