Draw mechanism for fischer esterification using the reagents benzoic acid, methanol and sulfuric acid using the general mechanism attached as reference. HINT: You just need to replace the Rs in the general mechanism for the actual groups in your reagents.
Draw mechanism for fischer esterification using the reagents benzoic acid, methanol and sulfuric acid using the general mechanism attached as reference. HINT: You just need to replace the Rs in the general mechanism for the actual groups in your reagents.
Chapter10: Organohalides
Section10.SE: Something Extra
Problem 19MP: The formation of Br2 from NBS first involves the reaction of NBS with HBr to form an iminol...
Related questions
Question
100%
Draw mechanism for fischer esterification using the reagents benzoic acid, methanol and sulfuric acid using the general mechanism attached as reference.
HINT: You just need to replace the Rs in the general mechanism for the actual groups in your reagents.
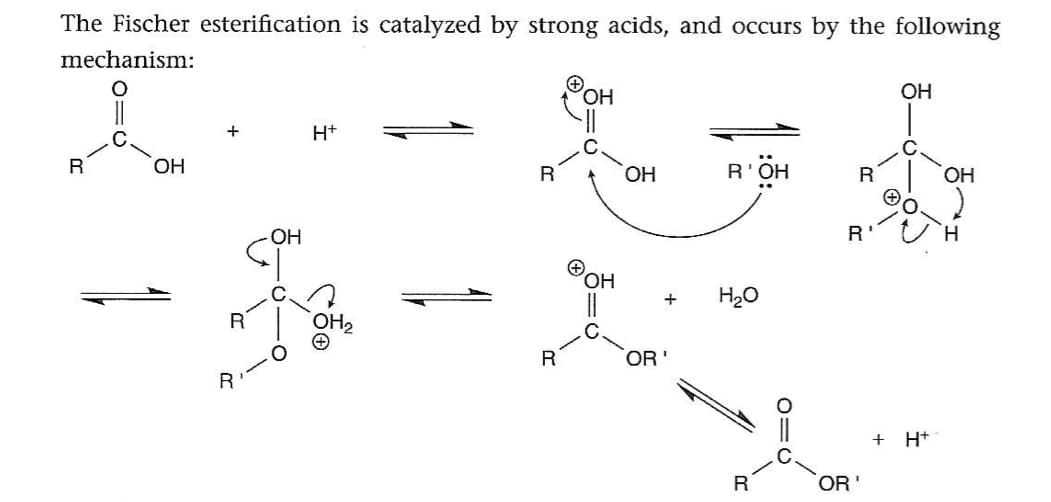
Transcribed Image Text:The Fischer esterification is catalyzed by strong acids, and occurs by the following
mechanism:
HO,
OH
H+
R
HO,
R
HO,
R'OH
R
ОН
ОН
R
+
R
OR'
+ H+
R
OR'
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning