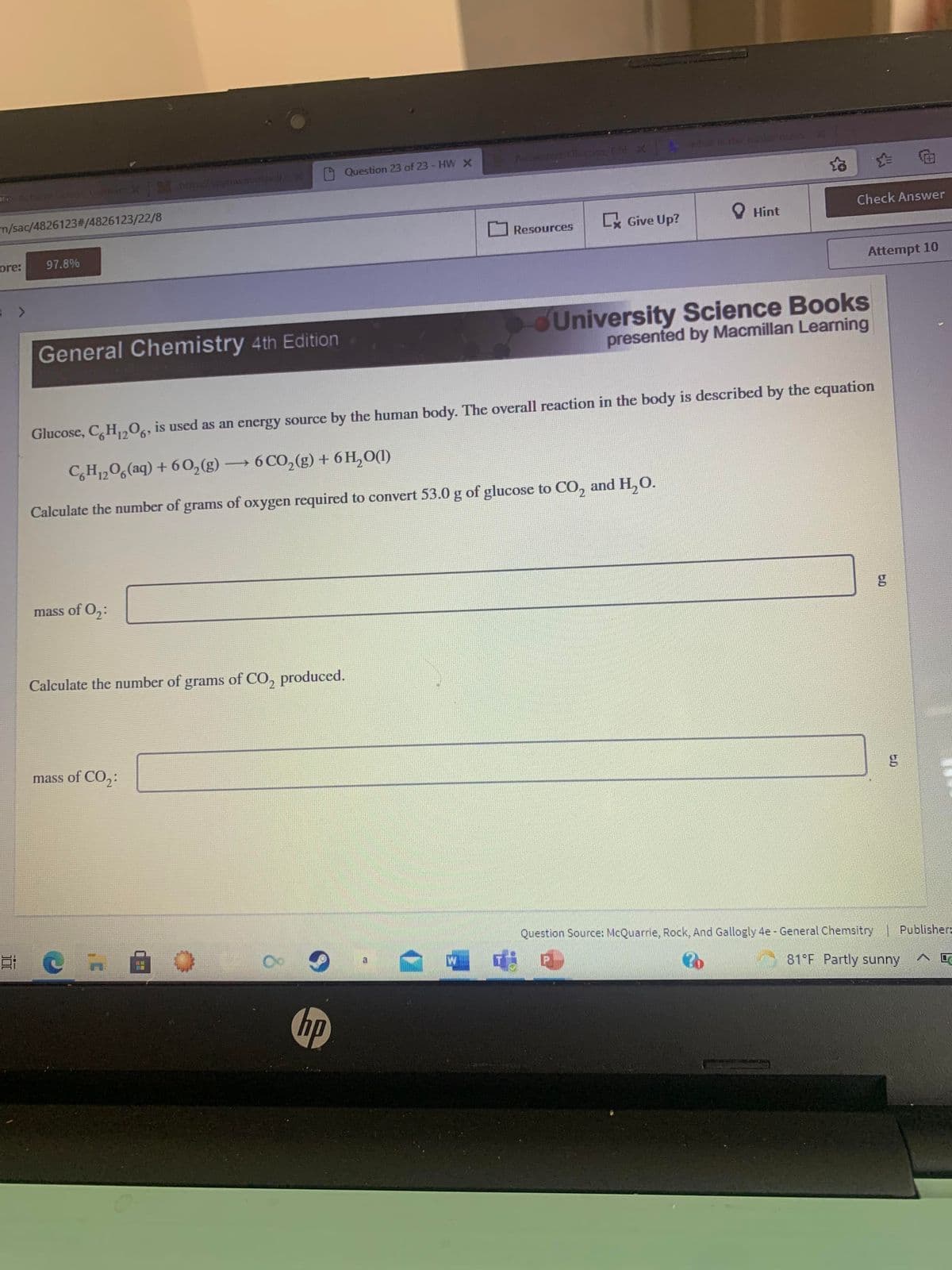
dUniversity Science Books presented by Macmillan Learning General Chemistry 4th Edition Glucose, C,H,,0,, is used as an energy source by the human body. The overall reaction in the body is described by the equation CH,0,(aq) + 6 0,(g) 6 CO,(g) + 6 H,O(1) Calculate the number of grams of oxygen required to convert 53.0 g of glucose to CO, and H,O. mass of O,: Calculate the number of grams of CO, produced. mass of CO,:
dUniversity Science Books presented by Macmillan Learning General Chemistry 4th Edition Glucose, C,H,,0,, is used as an energy source by the human body. The overall reaction in the body is described by the equation CH,0,(aq) + 6 0,(g) 6 CO,(g) + 6 H,O(1) Calculate the number of grams of oxygen required to convert 53.0 g of glucose to CO, and H,O. mass of O,: Calculate the number of grams of CO, produced. mass of CO,:
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter24: Biochemistry
Section: Chapter Questions
Problem 34GQ: The first step of the metabolic process known as glycolysis is the conversion of glucose to glucose-...
Related questions
Question
Transcribed Image Text:9 Question 23 of 23 - HWX
m/sac/4826123%2#/4826123/22/8
Check Answer
R Give Up?
O Hint
Resources
ore:
97.8%
Attempt 10
University Science Books
presented by Macmillan Learning
General Chemistry 4th Edition
Glucose, C,H,,06, is used as an energy source by the human body. The overall reaction in the body is described by the equation
C,H,06(aq) + 60,(g)
→6 CO,(g) + 6 H,0(1)
|
Calculate the number of grams of oxygen required to convert 53.0 g of glucose to CO, and H,O.
mass of O,:
Calculate the number of grams of CO, produced.
mass of CO,:
Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemsitry Publisher:
81 F Partly sunny
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning



Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning