e Web Note made in Microsoft Edge Chapter 2: EOc Question 3 [References) 1 pt An atom has a very small nucleus surrounded by an electron "cloud." The figure below represents the nucleus with a diameter of about 2 mm and describes the electron cloud as extending over 200 m. If the diameter of an atom is 1 x 10-8 cm, what is the approximate diameter of its nucleus? %3D Question 4 1 pt Question 5 1 pt Nucleus with protons (positive electric charge) and neutrons (no electric charge). Question 6 1 pt Question 7 1 pt Question 8 1 pt Question 9 1 pt Question 10 1 pt Question 11 1 pt Question 12 1 pt Question 13 1 pt Electrons (negative electric charge). The number of electrons and protons is equal in an electrically neutral atom. Question 14 1 pt Question 15 1 pt Diameter = 2 cm Question 16 1 pt Question 17 1 pt Submit Answer Try Another Version 10 item attempts remaining Question 18 1 pt Progress: 2/25 items Due May 13 at 08:55 PM Previous Next Finish Assignment Email Instructor Save and Exit Cengage Learning | Cengage Technical Support e Web Note made in Microsoft Edge Chapter 2: EOC [References) Question 1 1 pt Give the complete symbol (X), including atomic number and mass number, of Question 2 1 pt a. a manganese atom with 27 neutrons. Question 3 1 pt 4X: Question 4 1 pt b. a plutonium atom with 144 neutrons. Question 5 1 pt X: Question 6 1 pt Question 7 1 pt c. a tantalum atom with 108 neutrons. Question 8 1 pt X: Question 9 1 pt Question 10 1 pt Submit Answer Try Another Version 10 item attempts remaining Question 11 1 pt Question 12 1 pt Question 13 1 pt Question 14 1 pt Question 15 1 pt Question 16 1 pt Progress: 2/25 items Due May 13 at 08:55 PM Next Previous Finish Assignment Email Instructor Save and Exit Cengage Learning | Cengage Technical Support
e Web Note made in Microsoft Edge Chapter 2: EOc Question 3 [References) 1 pt An atom has a very small nucleus surrounded by an electron "cloud." The figure below represents the nucleus with a diameter of about 2 mm and describes the electron cloud as extending over 200 m. If the diameter of an atom is 1 x 10-8 cm, what is the approximate diameter of its nucleus? %3D Question 4 1 pt Question 5 1 pt Nucleus with protons (positive electric charge) and neutrons (no electric charge). Question 6 1 pt Question 7 1 pt Question 8 1 pt Question 9 1 pt Question 10 1 pt Question 11 1 pt Question 12 1 pt Question 13 1 pt Electrons (negative electric charge). The number of electrons and protons is equal in an electrically neutral atom. Question 14 1 pt Question 15 1 pt Diameter = 2 cm Question 16 1 pt Question 17 1 pt Submit Answer Try Another Version 10 item attempts remaining Question 18 1 pt Progress: 2/25 items Due May 13 at 08:55 PM Previous Next Finish Assignment Email Instructor Save and Exit Cengage Learning | Cengage Technical Support e Web Note made in Microsoft Edge Chapter 2: EOC [References) Question 1 1 pt Give the complete symbol (X), including atomic number and mass number, of Question 2 1 pt a. a manganese atom with 27 neutrons. Question 3 1 pt 4X: Question 4 1 pt b. a plutonium atom with 144 neutrons. Question 5 1 pt X: Question 6 1 pt Question 7 1 pt c. a tantalum atom with 108 neutrons. Question 8 1 pt X: Question 9 1 pt Question 10 1 pt Submit Answer Try Another Version 10 item attempts remaining Question 11 1 pt Question 12 1 pt Question 13 1 pt Question 14 1 pt Question 15 1 pt Question 16 1 pt Progress: 2/25 items Due May 13 at 08:55 PM Next Previous Finish Assignment Email Instructor Save and Exit Cengage Learning | Cengage Technical Support
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter2: Atoms, Molescules, And Ions
Section: Chapter Questions
Problem 2.55QP: While traveling to a distant universe, you discover the hypothetical element X. You obtain a...
Related questions
Question
Transcribed Image Text:e Web Note made in Microsoft Edge
Chapter 2: EOc
Question 3
[References)
1 pt
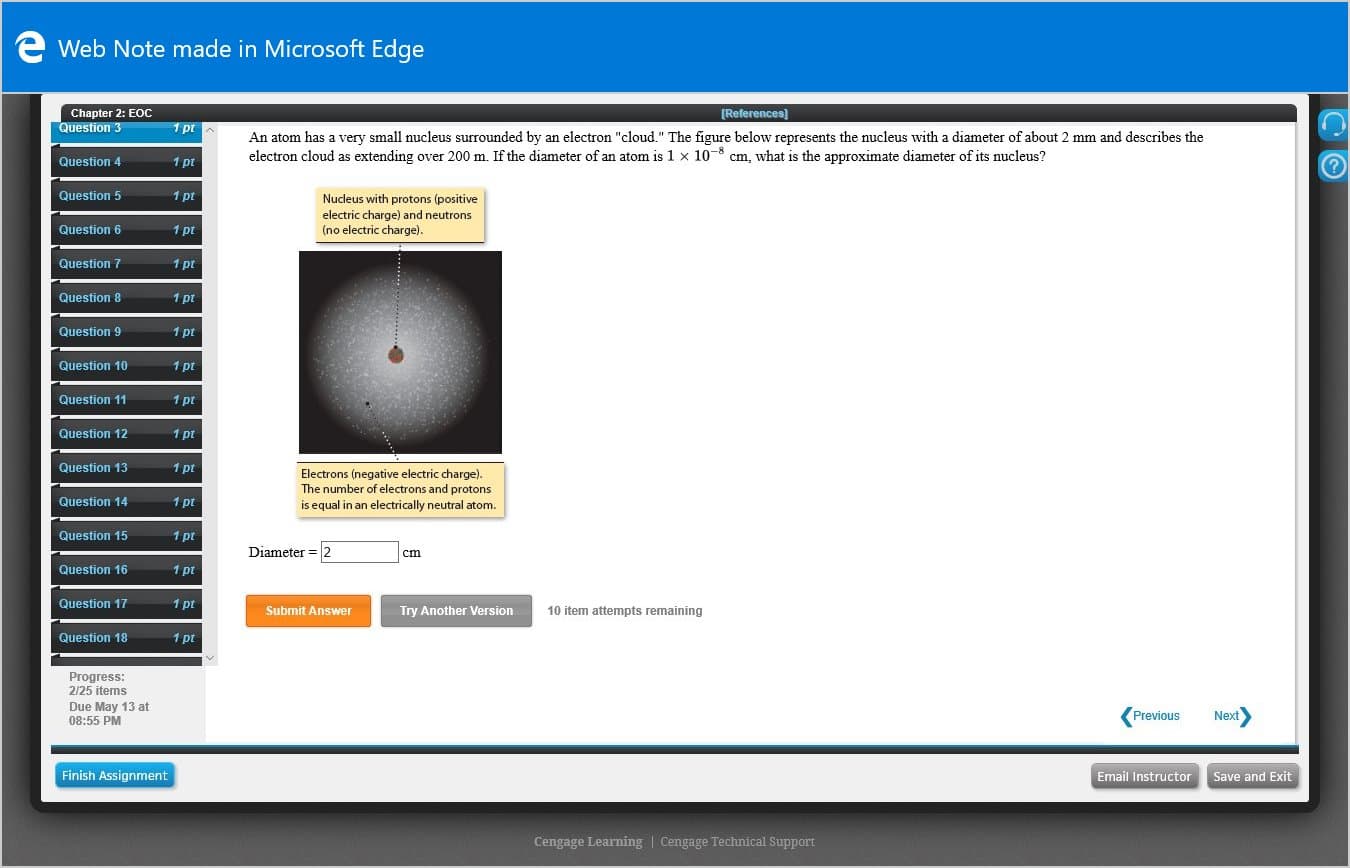
An atom has a very small nucleus surrounded by an electron "cloud." The figure below represents the nucleus with a diameter of about 2 mm and describes the
electron cloud as extending over 200 m. If the diameter of an atom is 1 x 10-8 cm, what is the approximate diameter of its nucleus?
%3D
Question 4
1 pt
Question 5
1 pt
Nucleus with protons (positive
electric charge) and neutrons
(no electric charge).
Question 6
1 pt
Question 7
1 pt
Question 8
1 pt
Question 9
1 pt
Question 10
1 pt
Question 11
1 pt
Question 12
1 pt
Question 13
1 pt
Electrons (negative electric charge).
The number of electrons and protons
is equal in an electrically neutral atom.
Question 14
1 pt
Question 15
1 pt
Diameter = 2
cm
Question 16
1 pt
Question 17
1 pt
Submit Answer
Try Another Version
10 item attempts remaining
Question 18
1 pt
Progress:
2/25 items
Due May 13 at
08:55 PM
Previous
Next
Finish Assignment
Email Instructor Save and Exit
Cengage Learning | Cengage Technical Support
Transcribed Image Text:e Web Note made in Microsoft Edge
Chapter 2: EOC
[References)
Question 1
1 pt
Give the complete symbol (X), including atomic number and mass number, of
Question 2
1 pt
a. a manganese atom with 27 neutrons.
Question 3
1 pt
4X:
Question 4
1 pt
b. a plutonium atom with 144 neutrons.
Question 5
1 pt
X:
Question 6
1 pt
Question 7
1 pt
c. a tantalum atom with 108 neutrons.
Question 8
1 pt
X:
Question 9
1 pt
Question 10
1 pt
Submit Answer
Try Another Version
10 item attempts remaining
Question 11
1 pt
Question 12
1 pt
Question 13
1 pt
Question 14
1 pt
Question 15
1 pt
Question 16
1 pt
Progress:
2/25 items
Due May 13 at
08:55 PM
Next
Previous
Finish Assignment
Email Instructor Save and Exit
Cengage Learning | Cengage Technical Support
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning