Energy of the emitted photons When an excited electron falls to a lower energy level it emits a photon. The energy of the emitted photon (E = hf) depends on the difference in energy between the two levels: hf = Ez - E Worked example: Refer to Figure 11.36. Consider an electron transition from energy level 6 to energy level 2 a) Calculate the energy of the photon that is emitted.
Energy of the emitted photons When an excited electron falls to a lower energy level it emits a photon. The energy of the emitted photon (E = hf) depends on the difference in energy between the two levels: hf = Ez - E Worked example: Refer to Figure 11.36. Consider an electron transition from energy level 6 to energy level 2 a) Calculate the energy of the photon that is emitted.
Foundations of Astronomy (MindTap Course List)
14th Edition
ISBN:9781337399920
Author:Michael A. Seeds, Dana Backman
Publisher:Michael A. Seeds, Dana Backman
Chapter7: Atoms And Spectra
Section: Chapter Questions
Problem 1LTL
Related questions
Question
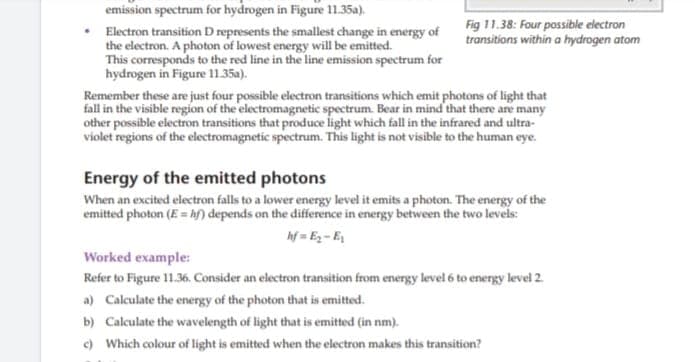
Transcribed Image Text:emission spectrum for hydrogen in Figure 11.35a).
Fig 11.38: Four possible electron
transitions within a hydrogen atom
Electron transition D represents the smallest change in energy of
the electron. A photon of lowest energy will be emitted.
This corresponds to the red line in the line emission spectrum for
hydrogen in Figure 11.35a).
Remember these are just four possible electron transitions which emit photons of light that
fall in the visible region of the electromagnetic spectrum. Bear in mind that there are many
other possible electron transitions that produce light which fall in the infrared and ultra-
violet regions of the electromagnetic spectrum. This light is not visible to the human eye.
Energy of the emitted photons
When an excited electron falls to a lower energy level it emits a photon. The energy of the
emitted photon (E = hf) depends on the difference in energy between the two levels:
hf = Ez- E
Worked example:
Refer to Figure 11.36. Consider an electron transition from energy level 6 to energy level 2.
a) Calculate the energy of the photon that is emitted.
b) Calculate the wavelength of light that is emitted (in nm).
e) Which colour of light is emitted when the electron makes this transition?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:
9781337399920
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning


Stars and Galaxies (MindTap Course List)
Physics
ISBN:
9781337399944
Author:
Michael A. Seeds
Publisher:
Cengage Learning

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:
9781337399920
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning


Stars and Galaxies (MindTap Course List)
Physics
ISBN:
9781337399944
Author:
Michael A. Seeds
Publisher:
Cengage Learning