Enter your answer in scientific notation. Be sure to answer all parts. Nuclear waste disposal is one of the major concerns of the nuclear industry. In choosing a safe and stable environment to store nuclear wastes, consideration must be given to the heat released during nuclear decay. As an example, consider the ß-decay of 90Sr (89.907738 amu): 90 90 Sr - Y + 38 12= 28.1 yr 39 The 0Y (89.907152 amu) further decays as follows: 90 90 Y + Zr + B 40 12 = 64 hr 39 -1 Zirconium-90 (89.904703 amu) is a stable isotope. Beginning with one mole of "Sr, calculate the amount of heat released (in kilojoules) in one year corresponding to the number of moles of 0Sr decayed to "Zr. x 10 kJ
Enter your answer in scientific notation. Be sure to answer all parts. Nuclear waste disposal is one of the major concerns of the nuclear industry. In choosing a safe and stable environment to store nuclear wastes, consideration must be given to the heat released during nuclear decay. As an example, consider the ß-decay of 90Sr (89.907738 amu): 90 90 Sr - Y + 38 12= 28.1 yr 39 The 0Y (89.907152 amu) further decays as follows: 90 90 Y + Zr + B 40 12 = 64 hr 39 -1 Zirconium-90 (89.904703 amu) is a stable isotope. Beginning with one mole of "Sr, calculate the amount of heat released (in kilojoules) in one year corresponding to the number of moles of 0Sr decayed to "Zr. x 10 kJ
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter14: Nuclear Chemistry
Section: Chapter Questions
Problem 14.92PAE
Related questions
Question
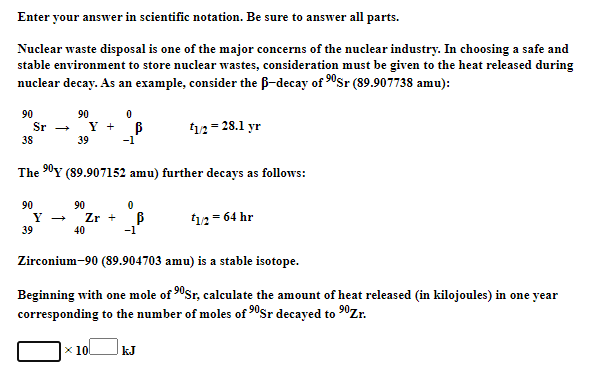
Transcribed Image Text:Enter your answer in scientific notation. Be sure to answer all parts.
Nuclear waste disposal is one of the major concerns of the nuclear industry. In choosing a safe and
stable environment to store nuclear wastes, consideration must be given to the heat released during
nuclear decay. As an example, consider the B-decay of 0sr (89.907738 amu):
90
Sr
90
Y + B
t12= 28.1 yr
38
39
-1
The 90Y (89.907152 amu) further decays as follows:
90
90
Zr +
40
12= 64 hr
39
-1
Zirconium-90 (89.904703 amu) is a stable isotope.
Beginning with one mole of 90Sr, calculate the amount of heat released (in kilojoules) in one year
corresponding to the number of moles of 90$r decayed to 0Zr.
x 10
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
