Ernest Rutherford carried out the first artificial transmutation in 1919 when he bombarded "N with alpha particles. The result was an oxygen nucleus: "N+He → °F* →"0+p Radiochemists use a shorthand notation to represent transmutations. Omitting the intermediate F* nucleus, the reaction above can be represented as 14N (a, p) 170. Write balanced equations for each of the indicated nuclear bombardments. (Use the lowest possible coefficients. Omit states of matter.) a. 27 Al (p, y) 28 Si b. 48 Ti (n, 8-) 4º v
Ernest Rutherford carried out the first artificial transmutation in 1919 when he bombarded "N with alpha particles. The result was an oxygen nucleus: "N+He → °F* →"0+p Radiochemists use a shorthand notation to represent transmutations. Omitting the intermediate F* nucleus, the reaction above can be represented as 14N (a, p) 170. Write balanced equations for each of the indicated nuclear bombardments. (Use the lowest possible coefficients. Omit states of matter.) a. 27 Al (p, y) 28 Si b. 48 Ti (n, 8-) 4º v
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter18: The Nucleus: A Chemist's View
Section: Chapter Questions
Problem 87IP
Related questions
Question
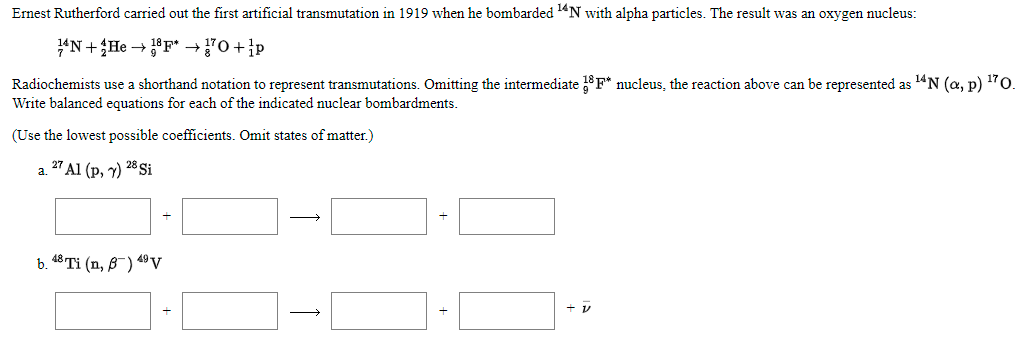
Transcribed Image Text:Ernest Rutherford carried out the first artificial transmutation in 1919 when he bombarded "N with alpha particles. The result was an oxygen nucleus:
"N+He → °F* →"0+p
Radiochemists use a shorthand notation to represent transmutations. Omitting the intermediate F* nucleus, the reaction above can be represented as 14N (a, p) 170.
Write balanced equations for each of the indicated nuclear bombardments.
(Use the lowest possible coefficients. Omit states of matter.)
a. 27 Al (p, y) 28 Si
b. 48 Ti (n, 8") 4º v
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning