Essential oils are concentrated liquid containing metabolites extracted from plant sources and are widely used in the production of perfumes. The structures of some of these compounds present in essential oils, along with acetone, are presented below. CH3 H2C CH H3CO. CH2 CH H2C. CH2 H2C CH CH2 Но H3C CH2 Compound A MM: 164.2 g/mol Boiling Point: 254 C Compound B MM: 162.19 g/mol Boiling Point: 232 C Compound C MM: 136.24 g/mol Boiling Point: 176 C 1. Determine the most possible boiling point of each of the compounds. Refer to the possible boiling points listed above. 2. An equal volume of COMPOUNDS A, B and C were stored in separate beakers which were left uncovered at room temperature. If the three liquids were allowed to stand for about 10 minutes, which of the following will have the least amount of liquid remaining in the container? b. а. What physical property is the basis of the answer? c. Among the three liquids, what is the most recommended ingredient of a perfume that will have the most lasting scent?
Essential oils are concentrated liquid containing metabolites extracted from plant sources and are widely used in the production of perfumes. The structures of some of these compounds present in essential oils, along with acetone, are presented below. CH3 H2C CH H3CO. CH2 CH H2C. CH2 H2C CH CH2 Но H3C CH2 Compound A MM: 164.2 g/mol Boiling Point: 254 C Compound B MM: 162.19 g/mol Boiling Point: 232 C Compound C MM: 136.24 g/mol Boiling Point: 176 C 1. Determine the most possible boiling point of each of the compounds. Refer to the possible boiling points listed above. 2. An equal volume of COMPOUNDS A, B and C were stored in separate beakers which were left uncovered at room temperature. If the three liquids were allowed to stand for about 10 minutes, which of the following will have the least amount of liquid remaining in the container? b. а. What physical property is the basis of the answer? c. Among the three liquids, what is the most recommended ingredient of a perfume that will have the most lasting scent?
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.103P
Related questions
Question
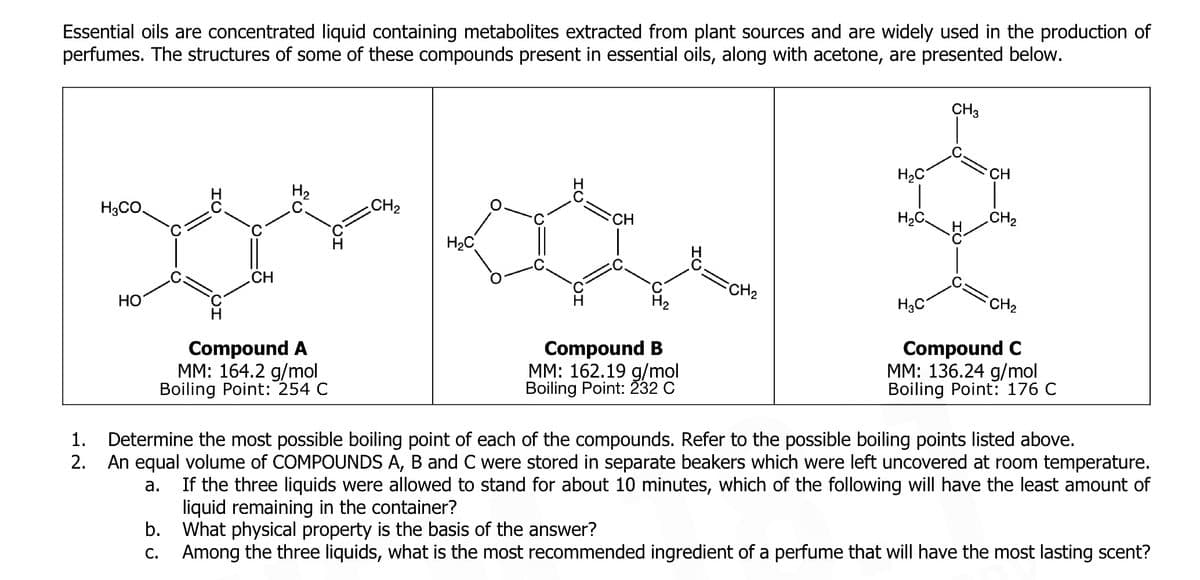
Transcribed Image Text:Essential oils are concentrated liquid containing metabolites extracted from plant sources and are widely used in the production of
perfumes. The structures of some of these compounds present in essential oils, along with acetone, are presented below.
CH3
H2C
CH
H2
H3CO,
CH2
CH
H2C
H2C
CH
FCH2
HO
H3C
CH2
Compound A
MM: 164.2 g/mol
Boiling Point: 254 C
Compound B
MM: 162.19 g/mol
Boiling Point: 232 C
Compound C
MM: 136.24 g/mol
Boiling Point: 176 C
Determine the most possible boiling point of each of the compounds. Refer to the possible boiling points listed above.
An equal volume of COMPOUNDS A, B and C were stored in separate beakers which were left uncovered at room temperature.
1.
2.
If the three liquids were allowed to stand for about 10 minutes, which of the following will have the least amount of
liquid
b. What physical property is the basis of the answer?
Among the three liquids, what is the most recommended ingredient of a perfume that will have the most lasting scent?
а.
maining in
container?
С.
IU
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax