Example 3.6 يخلط معدن النحاس والفضة جيدا في شكل مسحوق ، بنسب 80 وزن / س نحاس و 20 ث / س Ag ويتم تسخينه إلى درجة حرارة أعلى بكثير من درجة حرارة انصهار السبيكة. ثم يبرد المعدن السائل ويسمح له بالوصول إلى توازن ديناميكي حراري. أعط تكوين كل مرحلة والمقدار النسبي لكل مرحلة Copper and silver metal are mixed thoroughly in powder form, in proportions 80 w/o Cu and 20 w/o Ag and heated to well above the melting temperature of the alloy. The liquid metal is then cooled and allowed to reach thermodynamic equilibrium. Give the composition of each phase and the relative amount of each phase: a. At 1,000°C. Answer c. At 700°C. b. At 7800C. 1100 1083 Liquid (L) 980.5" 900 B+L a+L 8.8 28.1 92 700 500 50 100 Cu Temperature (5.)
Example 3.6 يخلط معدن النحاس والفضة جيدا في شكل مسحوق ، بنسب 80 وزن / س نحاس و 20 ث / س Ag ويتم تسخينه إلى درجة حرارة أعلى بكثير من درجة حرارة انصهار السبيكة. ثم يبرد المعدن السائل ويسمح له بالوصول إلى توازن ديناميكي حراري. أعط تكوين كل مرحلة والمقدار النسبي لكل مرحلة Copper and silver metal are mixed thoroughly in powder form, in proportions 80 w/o Cu and 20 w/o Ag and heated to well above the melting temperature of the alloy. The liquid metal is then cooled and allowed to reach thermodynamic equilibrium. Give the composition of each phase and the relative amount of each phase: a. At 1,000°C. Answer c. At 700°C. b. At 7800C. 1100 1083 Liquid (L) 980.5" 900 B+L a+L 8.8 28.1 92 700 500 50 100 Cu Temperature (5.)
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
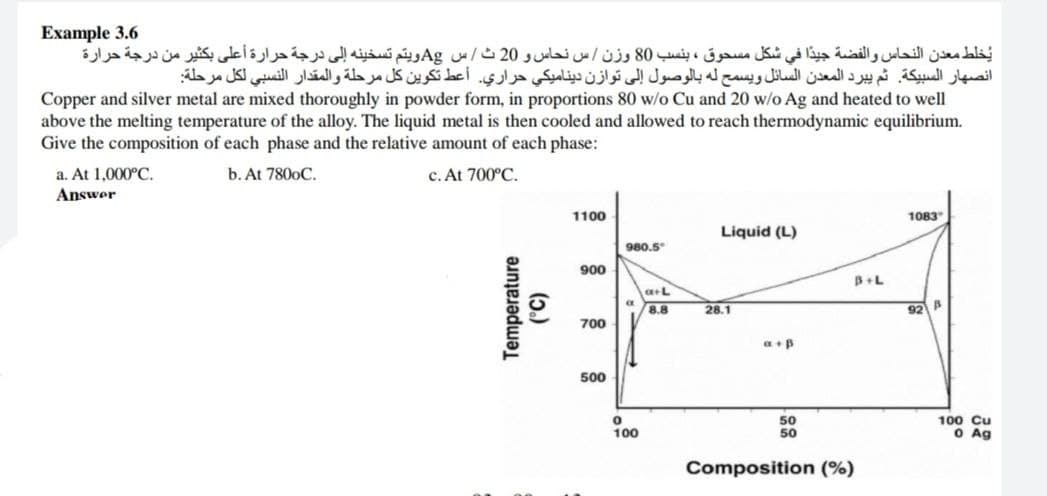
Transcribed Image Text:Example 3.6
يخلط معدن النحاس والفضة جيدا في شكل مسحوق ، بنسب 80 وزن / س نحاس و 20 ث / س Ag ويتم تسخينه إلى درجة حرارة أعلى بكثير من درجة حرارة
انصهار السبيكة. ثم يبرد المعدن السائل ويسمح له بالوصول إلى توازن دیناميكي حراري. أعط تكوين كل مرحلة والمقدار النسبي لكل مرحلة
Copper and silver metal are mixed thoroughly in powder form, in proportions 80 w/o Cu and 20 w/o Ag and heated to well
above the melting temperature of the alloy. The liquid metal is then cooled and allowed to reach thermodynamic equilibrium.
Give the composition of each phase and the relative amount of each phase:
a. At 1,000°C.
b. At 7800C.
c. At 700°C.
Answer
1100
1083
Liquid (L)
980.5
Je
900
a+L
8.8
28.1
92 A
700
a+B
500
50
50
100 Cu
O Ag
100
Composition (%)
Temperature
(5.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY