Exercise 1 Fig. (1) is a PV diagram for a reversible heat engine in whicth 1.0 mol of argon nearly ideal monoatomic gas is initially at STP (point a). Points b and c are on an isotherm at T 423K. Process ab is at constant volume, process ac at constant pressure. (a) Is the path of the cycle carried out clockwise or counterclockwise? (b) What is the efficiency of the engine? Points b a T- 423 volum pre T-423 K
Exercise 1 Fig. (1) is a PV diagram for a reversible heat engine in whicth 1.0 mol of argon nearly ideal monoatomic gas is initially at STP (point a). Points b and c are on an isotherm at T 423K. Process ab is at constant volume, process ac at constant pressure. (a) Is the path of the cycle carried out clockwise or counterclockwise? (b) What is the efficiency of the engine? Points b a T- 423 volum pre T-423 K
Chapter4: The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 86CP: A cylinder contains 500 g of helium at 120 atm and 20 . The valve is leaky, and all the gas slowly...
Related questions
Question
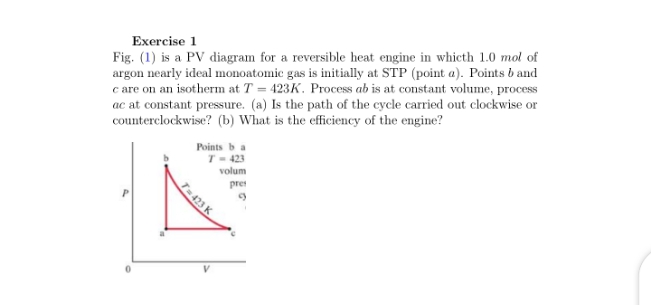
Transcribed Image Text:Exercise 1
Fig. (1) is a PV diagram for a reversible heat engine in whicth 1.0 mol of
argon nearly ideal monoatomic gas is initially at STP (point a). Points b and
c are on an isotherm at T = 423K. Process ab is at constant volume, process
ac at constant pressure. (a) Is the path of the cycle carried out clockwise or
counterclockwise? (b) What is the efficiency of the engine?
Points b a
T- 423
volum
pre
T-423 K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College