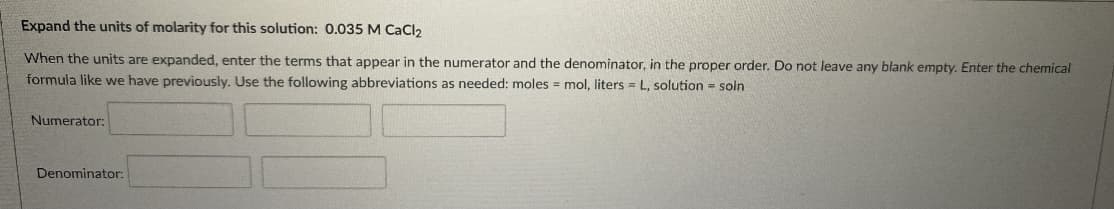
Expand the units of molarity for this solution: 0.035 M CaCI2 When the units are expanded, enter the terms that appear in the numerator and the denominator, in the proper order. Do not leave any blank empty. Enter the chemical formula like we have previously. Use the following abbreviations as needed: moles = mol, liters L, solution soln Numerator: Denominator:
For 2.56 g of succinic acid in 500. mL of water:
The molality of the solution:
Molality = #mole solute/kg solvent:
With a density of water of 1.00 g/cm3, 500. mL = 0.500 kg
Molality = 0.0217 mol
0.500 kg = 0.0434 molal
The mole fraction of succinic acid in the solution:
For mole fraction we need both the # moles of solute and #moles of solvent.
Moles of water = 500. g H2O •
1 mol H2O
18.02 g H2O
= 27.7 mol H2O
The mf of acid = 0.0217 mol
(0.0217 mol + 27.7 mol) = 7.81 x 10-4
The weight percentage of succinic acid in the solution:
The fraction of total mass of solute + solvent which is solute:
Weight percentage = 2.56 g succinic acid
502.56 g acid + water • 100 = 0.509% succinic acid
3. Complete the following transformations for
NaI:
Weight percent:
0.15 mol NaI
1 kg solvent
•
0.15 mol NaI
1 kg solvent
=
22.5 g NaI
1 kg solvent
22.5 g NaI
1000 g solvent + 22.5 g NaI • 100 = 2.2 % NaI
Mole fraction:
1000 g H2O = 55.51 mol H2O
XNaI =
0.15 mol NaI
55.51 mol H2O + 0.15 mol NaI
= 2.7 x 10-3
Chapter 14 Solutions and Their Behavior
201
C2H5OH:
Molality:
5.0 g C2H5OH
100 g solution
•
1 mol C2H5OH
46.07 g C2H5OH
• 100 g solution
95 g solvent
• 1000 g solvent
1 kg solvent
= 1.1 molal
Mole fraction:
5.0 g C2H5OH
1 •
1 mol C2H5OH
46.07 g C2H5OH = 0.11 mol C2H5OH
and for water:
95 g H2O
1 •
1 mol H2O
18.02 g H2O = 5.27 mol H2O
XC2H5
OH =
0.11 mol C2H5OH
5.27 mol H2O + 0.11 mol C2H5OH
= 0.020
C12H22O11:
Weight percent:
0.15 mol C12H22O11
1 kg solvent •
342.3 g C12H22O11
1 mol C12H22O11 =
51.3 g C12H22O11
1 kg solvent
51.3 g C12H22O11
1000 g H2O + 51.3 g C12H22O11 x 100 = 4.9 % C12H22O11
Mole fraction:
XC12H22O11 =
0.15 mol C12H22O11
55.51 mol H2O + 0.15 mol C12H22O11
= 2.7 x 10-3
5. To prepare a solution that is 0.200 m Na2CO3:
0.200 mol Na 2CO3
1 kg H2O
• 0.125 kg H2O
1
• 106.0 g Na 2CO3
1 mol Na 2CO3
= 2.65 g Na2CO3
mol Na2CO3 =
0.200 mol Na 2CO3
1 kg H2O
• 0.125 kg H2O
1
= 0.025 mol
The mole fraction of Na2CO3 in the resulting solution:
125. g H2O
1 •
1 mol H2O
18.02 g H2O = 6.94 mol H2O
XNa2
CO3
=
0.025 mol Na 2CO3
0.025 mol Na 2CO3 + 6.94 mol H2O
= 3.59 x 10-3
7. To calculate the number of mol of C3H5(OH)3:
0.093 =
x mol C3H5 (OH)3
x mol C3H5 (OH)3 + (425 g H2Oi 1 mol H2O
18.02 g H2O
Chapter 14 Solutions and Their Behavior
202
0.093 =
x mol C3H5(OH)3
x mol C3H5(OH)3 + 23.58 mol H2O
0.093(x + 23.58) = x and solving for x we get 2.4 mol C3H5(OH)3
Grams of glycerol needed: 2.4 mol C3H5(OH)3 •
92.1 g
1 mol = 220 g C3H5(OH)3
The molality of the solution is (2.4 mol C3H5(OH)3, 0.425 kg H2O)= 5.7 m
9. Concentrated HCl is 12.0M and has a density of 1.18 g/cm3.
(a) The molality of the solution:
Molality is defined as moles HCl/kg solvent, so begin by deciding the mass of 1 L, and the
mass of water in that 1L. Since the density = 1.18g/mL, then 1 L (1000 mL) will have a
mass of 1180g.
The mass of HCl present in 12.0 mol HCl =
12.0 mol HCl • 36.46 g HCl
1 mol HCl = 437.52 g HCl
Since 1 L has a mass of 1180 g and 437.52 g is HCl, the difference (1180-437.52) is
solvent. So 1 L has 742.98 g water.
12.0 mol HCl
1 L • 1 L
742.98 g H2O •
1000 g H2O
1 kg H2O = 16.2 m
(b) Weight percentage of HCl:
12.0 mol HCl has a mass of 437.52 g, and the 1 L of solution has a mass of 1180 g.
%HCl =
437.52 g HCl
1180 g solution •100 = 37.1 %
11. The concentration of ppm expressed in grams is:
0.18 ppm = 0.18 g solute
1.0x106 g solvent
=
0.18 g solute
1.0x103 kg solvent
or
0.00018 g solute
1 kg water
0.00018 g Li +
1 kg water
•
1 mol Li
+
6.939 g Li
+ = 2.6 x 10-5 molal Li+
The Solution Process
13. Pairs of liquids that will be miscible:
(a) H2O/CH3CH2CH2CH3
Will not be miscible. Water is a polar substance, while butane is nonpolar.
(b) C6H6/CCl4
Will be miscible. Both liquids are nonpolar and are expected to be miscible
Chapter 14 Solutions and Their Behavior
203
(c) H2O/CH3CO2H
Will be miscible. Both substances can hydrogen bond, and we know that they mix—since a
5% aqueous solution of acetic acid is sold as "vinegar"
15. The enthalpy of solution for LiCl:
The process can be represented as LiCl (s) →LiCl (aq)
The ΔrH = ΣΔfH (product) - ΣΔfH (reactant)
= (-445.6 kJ/mol)(1mol) - (-408.7 kJ/mol)(1mol) = -36.9 kJ
The similar calculation for NaCl is + 3.9 kJ. Note that the enthalpy of solution for NaCl is
endothermic while that for LiCl is exothermic.
Note the data (-408.7 kJ/mol) is from Table 14.1.
17. Raising the temperature of the solution will increase the solubility of NaCl in water. To
increase the amount of dissolved NaCl in solution one must (c) raise the temperature of the
solution and add some NaCl.
Henry’s Law
19. Solubility of O2 = k • PO2
= (1.66x 10-6 M
mm Hg ) • 40 mm Hg = 6.6 x 10-5 M O2
and 6.6 x 10-5 mol
L
• 32.0 g O2
1 mol O2
= 2 x 10-3 g O2
L
21. Solubility = k • PCO2 ;0.0506 M = (4.48 x 10-5 M
mm Hg ) • PCO2
Step by step
Solved in 2 steps









