Experiment # 15 Acid Orange Introduction Dyes and pigments are among the earliest evidences of man's ability to take advantage of the chemical properties of materials. The range of man's chemical creativity is evidenced in early cave paintings, from the Han Purple used to color the Terracotta Army (210 BC), and the use of the purple dye derived from murex mollusks during the time of Alexander the Great. The first "modern" synthetic dye was mauveine, discovered by William Henry Perkin in in 1856 when he was only 18 years old, which he patented. His chemical discovery sparked the development of the chemical industry, fueled by the textile industry and coinciding with the industrial revolution in mid-1800s London. In this lab, a diazo dye will be prepared. The first azo dye was chrysoidine or London Yellow, made by coupling aniline to m-phenylenediamine, and has been used to dye wool since 1875. Diazo dyes are among the most widely-used synthetic colorants, with applications ranging from cosmetics to food coloring. Do you ever wonder why the mac and cheese in Panda House looks very bright? It is because of Yellow #5 and Yellow #6. Look up the structure of these two dyes and research their possible effects to human physiology. In this green experiment, the diazonium will be produced without the use of sulfuric acid, which as you already know, is highly corrosive. Production of the diazonium ion from an aryl amine requires a strong acid, in this case, the sulfanilic acid starting material provides just that from its sulfonic acid (ArSO3H) functionality. No solvent will be used in the reaction except for a drop of water, and the purification will be accomplished by washing off any remaining starting material with ethyl acetate. HO NH2 NaNO2 + SO3H SO3H N=N- OH NaO3S your Procedure Substance Sulfanilic acid 2-Naphthol NaNO2 Acid Orange Mass (g) MW (g/mol) Mmol 0.1 0.1 0.1 Equivalents To a dry mortar and pestle add the three starting materials and gently mix the solids. Do not pound dry nitrate salts as they pose a danger of explosion. Rather, use a circular motion to grind the materials. Add a very small drop of water using a glass pipette and then grind the materials slowly using circular motions to pulverize the crystals for 10 min. Note any observations. You should obtain a wet powder. If you obtained a paste, you have added too much water and would have to let the product dry in air before proceeding. Add ethyl acetate (5 mL) to the dry powder and continue to grind to dissolve any unreacted starting material. Assemble a vacuum filtration with a Hirsh funnel wetted with ethyl acetate. When you are ready, turn the vacuum on and transfer the reaction mixture using a glass pipette to the center of the filter paper. Use additional ethyl acetate (3 mL) to transfer the entirety of the product from the mortar, as well as to wash the product. Discard the filtrate in the organic waste bottle and let the precipitate air-dry for 15 min. The product is water soluble and somewhat hygroscopic, avoid its exposure to humidity. Weigh the solids to obtain the percent yield and determine its melting point. Note be that although the product was purified of the organic starting materials by the ethyl acetate wash, there may be unreacted sodium nitrite left. For the purposes of this lab, we will assume that all of the nitrite reacted. However, to fully purify the product, the dye may be washed with saturated sodium chloride solution followed by cold methanol. guir Characterization Obtain a UV-Vis spectrum of your product by dissolving 10 mg Acid Orange in 100 mL distilled water using a volumetric flask. Calculate the molarity of this solution. Next, take 10 mL of this solution and dilute it with 100 mL of water using a volumetric flask. Transfer the solutions in labeled beakers so others may use the volumetric flask. Use M1V1= M2V2 to calculate the concentration of the second solution. Follow instructions on how to run the UV-Vis spectrometer. Briefly, run a blank sample in water. Place your sample in a cuvette using a pipette. The reading on the y-axis must be between 0.2 - 1.0 absorption units, otherwise your sample is too dilute or concentrated. Note the maxima of the peaks observed, both in wavelength and absorption units (A). Calculate the molar absorptivity at the maximum wavelength (max) by using Beer's Law equation, & = A*b*c where & is the molar absoprtivity, A is the absortion, and c is the molar concentration. Of course, more than one concentration must be measured to get an accurate result. For the purposes of this lab, a single measurement will suffice. Print your spectrum and this attach to your laboratory report.
Experiment # 15 Acid Orange Introduction Dyes and pigments are among the earliest evidences of man's ability to take advantage of the chemical properties of materials. The range of man's chemical creativity is evidenced in early cave paintings, from the Han Purple used to color the Terracotta Army (210 BC), and the use of the purple dye derived from murex mollusks during the time of Alexander the Great. The first "modern" synthetic dye was mauveine, discovered by William Henry Perkin in in 1856 when he was only 18 years old, which he patented. His chemical discovery sparked the development of the chemical industry, fueled by the textile industry and coinciding with the industrial revolution in mid-1800s London. In this lab, a diazo dye will be prepared. The first azo dye was chrysoidine or London Yellow, made by coupling aniline to m-phenylenediamine, and has been used to dye wool since 1875. Diazo dyes are among the most widely-used synthetic colorants, with applications ranging from cosmetics to food coloring. Do you ever wonder why the mac and cheese in Panda House looks very bright? It is because of Yellow #5 and Yellow #6. Look up the structure of these two dyes and research their possible effects to human physiology. In this green experiment, the diazonium will be produced without the use of sulfuric acid, which as you already know, is highly corrosive. Production of the diazonium ion from an aryl amine requires a strong acid, in this case, the sulfanilic acid starting material provides just that from its sulfonic acid (ArSO3H) functionality. No solvent will be used in the reaction except for a drop of water, and the purification will be accomplished by washing off any remaining starting material with ethyl acetate. HO NH2 NaNO2 + SO3H SO3H N=N- OH NaO3S your Procedure Substance Sulfanilic acid 2-Naphthol NaNO2 Acid Orange Mass (g) MW (g/mol) Mmol 0.1 0.1 0.1 Equivalents To a dry mortar and pestle add the three starting materials and gently mix the solids. Do not pound dry nitrate salts as they pose a danger of explosion. Rather, use a circular motion to grind the materials. Add a very small drop of water using a glass pipette and then grind the materials slowly using circular motions to pulverize the crystals for 10 min. Note any observations. You should obtain a wet powder. If you obtained a paste, you have added too much water and would have to let the product dry in air before proceeding. Add ethyl acetate (5 mL) to the dry powder and continue to grind to dissolve any unreacted starting material. Assemble a vacuum filtration with a Hirsh funnel wetted with ethyl acetate. When you are ready, turn the vacuum on and transfer the reaction mixture using a glass pipette to the center of the filter paper. Use additional ethyl acetate (3 mL) to transfer the entirety of the product from the mortar, as well as to wash the product. Discard the filtrate in the organic waste bottle and let the precipitate air-dry for 15 min. The product is water soluble and somewhat hygroscopic, avoid its exposure to humidity. Weigh the solids to obtain the percent yield and determine its melting point. Note be that although the product was purified of the organic starting materials by the ethyl acetate wash, there may be unreacted sodium nitrite left. For the purposes of this lab, we will assume that all of the nitrite reacted. However, to fully purify the product, the dye may be washed with saturated sodium chloride solution followed by cold methanol. guir Characterization Obtain a UV-Vis spectrum of your product by dissolving 10 mg Acid Orange in 100 mL distilled water using a volumetric flask. Calculate the molarity of this solution. Next, take 10 mL of this solution and dilute it with 100 mL of water using a volumetric flask. Transfer the solutions in labeled beakers so others may use the volumetric flask. Use M1V1= M2V2 to calculate the concentration of the second solution. Follow instructions on how to run the UV-Vis spectrometer. Briefly, run a blank sample in water. Place your sample in a cuvette using a pipette. The reading on the y-axis must be between 0.2 - 1.0 absorption units, otherwise your sample is too dilute or concentrated. Note the maxima of the peaks observed, both in wavelength and absorption units (A). Calculate the molar absorptivity at the maximum wavelength (max) by using Beer's Law equation, & = A*b*c where & is the molar absoprtivity, A is the absortion, and c is the molar concentration. Of course, more than one concentration must be measured to get an accurate result. For the purposes of this lab, a single measurement will suffice. Print your spectrum and this attach to your laboratory report.
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.77PAE
Related questions
Question
Please fill in the table ( for experimental values imaginary numbers can be used)
Based on that can you calculate
% yield
Atom economy
Atom efficiency
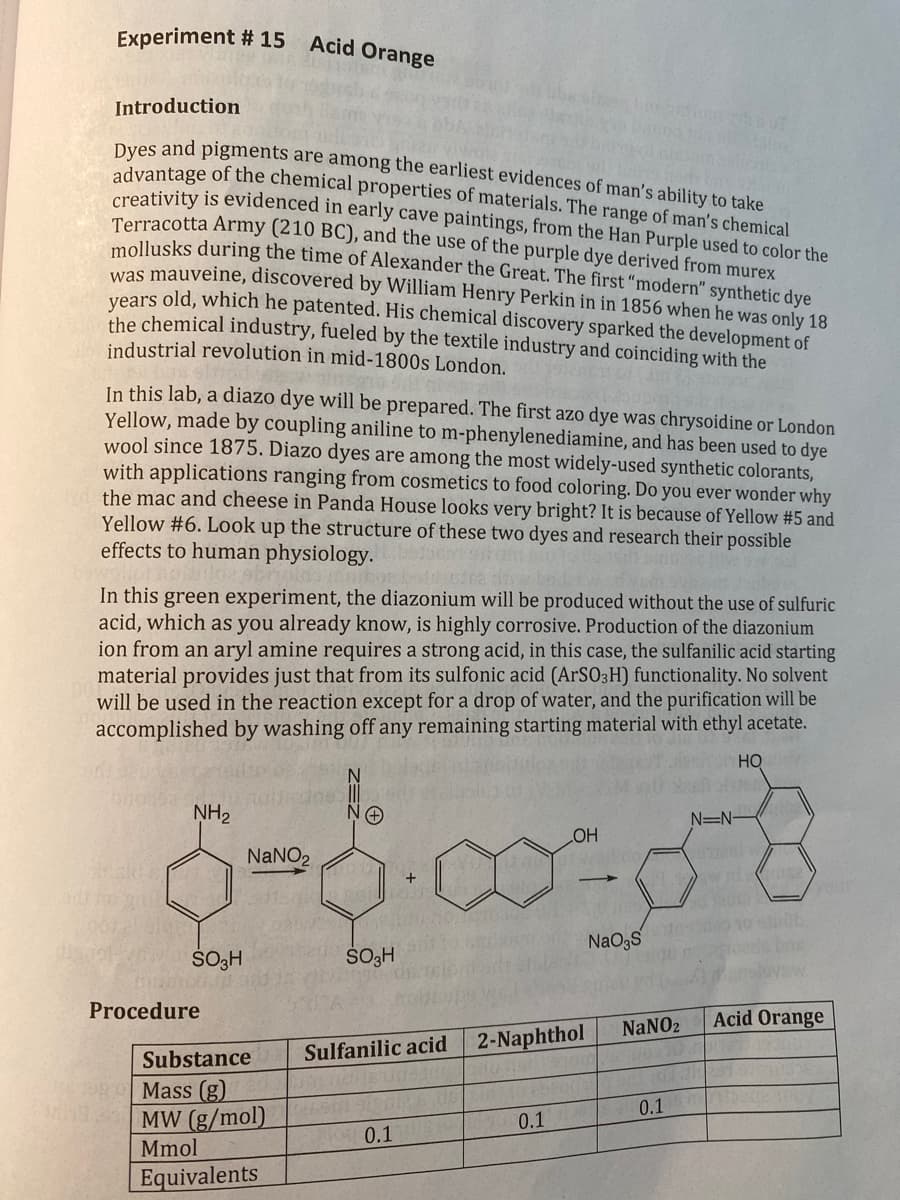
Transcribed Image Text:Experiment # 15
Acid Orange
Introduction
Dyes and pigments are among the earliest evidences of man's ability to take
advantage of the chemical properties of materials. The range of man's chemical
creativity is evidenced in early cave paintings, from the Han Purple used to color the
Terracotta Army (210 BC), and the use of the purple dye derived from murex
mollusks during the time of Alexander the Great. The first "modern" synthetic dye
was mauveine, discovered by William Henry Perkin in in 1856 when he was only 18
years old, which he patented. His chemical discovery sparked the development of
the chemical industry, fueled by the textile industry and coinciding with the
industrial revolution in mid-1800s London.
In this lab, a diazo dye will be prepared. The first azo dye was chrysoidine or London
Yellow, made by coupling aniline to m-phenylenediamine, and has been used to dye
wool since 1875. Diazo dyes are among the most widely-used synthetic colorants,
with applications ranging from cosmetics to food coloring. Do you ever wonder why
the mac and cheese in Panda House looks very bright? It is because of Yellow #5 and
Yellow #6. Look up the structure of these two dyes and research their possible
effects to human physiology.
In this green experiment, the diazonium will be produced without the use of sulfuric
acid, which as you already know, is highly corrosive. Production of the diazonium
ion from an aryl amine requires a strong acid, in this case, the sulfanilic acid starting
material provides just that from its sulfonic acid (ArSO3H) functionality. No solvent
will be used in the reaction except for a drop of water, and the purification will be
accomplished by washing off any remaining starting material with ethyl acetate.
HO
NH2
NaNO2
+
SO3H
SO3H
N=N-
OH
NaO3S
your
Procedure
Substance
Sulfanilic acid
2-Naphthol
NaNO2
Acid Orange
Mass (g)
MW (g/mol)
Mmol
0.1
0.1
0.1
Equivalents

Transcribed Image Text:To a dry mortar and pestle add the three starting materials and gently mix the
solids. Do not pound dry nitrate salts as they pose a danger of explosion. Rather, use
a circular motion to grind the materials. Add a very small drop of water using a glass
pipette and then grind the materials slowly using circular motions to pulverize the
crystals for 10 min. Note any observations. You should obtain a wet powder. If you
obtained a paste, you have added too much water and would have to let the product
dry in air before proceeding.
Add ethyl acetate (5 mL) to the dry powder and continue to grind to dissolve any
unreacted starting material. Assemble a vacuum filtration with a Hirsh funnel
wetted with ethyl acetate. When you are ready, turn the vacuum on and transfer the
reaction mixture using a glass pipette to the center of the filter paper. Use additional
ethyl acetate (3 mL) to transfer the entirety of the product from the mortar, as well
as to wash the product. Discard the filtrate in the organic waste bottle and let the
precipitate air-dry for 15 min. The product is water soluble and somewhat
hygroscopic, avoid its exposure to humidity.
Weigh the solids to obtain the percent yield and determine its melting point. Note
be that although the product was purified of the organic starting materials by the ethyl
acetate wash, there may be unreacted sodium nitrite left. For the purposes of this
lab, we will assume that all of the nitrite reacted. However, to fully purify the
product, the dye may be washed with saturated sodium chloride solution followed
by cold methanol.
guir Characterization
Obtain a UV-Vis spectrum of your product by dissolving 10 mg Acid Orange in 100
mL distilled water using a volumetric flask. Calculate the molarity of this solution.
Next, take 10 mL of this solution and dilute it with 100 mL of water using a
volumetric flask. Transfer the solutions in labeled beakers so others may use the
volumetric flask. Use M1V1= M2V2 to calculate the concentration of the second
solution.
Follow instructions on how to run the UV-Vis spectrometer. Briefly, run a blank
sample in water. Place your sample in a cuvette using a pipette. The reading on the
y-axis must be between 0.2 - 1.0 absorption units, otherwise your sample is too
dilute or concentrated. Note the maxima of the peaks observed, both in wavelength
and absorption units (A). Calculate the molar absorptivity at the maximum
wavelength (max) by using Beer's Law equation, & = A*b*c
where & is the molar absoprtivity, A is the absortion, and c is the molar
concentration. Of course, more than one concentration must be measured to get an
accurate result. For the purposes of this lab, a single measurement will suffice. Print
your spectrum and this attach to your laboratory report.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
