Experiment 2. Prepare one of the following solutions: (a) 100mL of aqueous 0.50% (w/v) NaCl, (b) 50mL of aqueous 0.25% (w/v) KCI, (c) 100mL of aqueous 0.50% (w/v) MgSO4, (d) 50mL of aqueous 0.25% (w/v) NaHCO3 1. Determine how many grams of solid are required. Show all steps for this calculation, using dimensional analysis with units clearly identified. 2. Prepare this aqueous solution, using a volumetric flask for the precise final volume measurement. Tap or distilled water may be used. 3. Clearly write the procedure (step by step) for making this solution.
Experiment 2. Prepare one of the following solutions: (a) 100mL of aqueous 0.50% (w/v) NaCl, (b) 50mL of aqueous 0.25% (w/v) KCI, (c) 100mL of aqueous 0.50% (w/v) MgSO4, (d) 50mL of aqueous 0.25% (w/v) NaHCO3 1. Determine how many grams of solid are required. Show all steps for this calculation, using dimensional analysis with units clearly identified. 2. Prepare this aqueous solution, using a volumetric flask for the precise final volume measurement. Tap or distilled water may be used. 3. Clearly write the procedure (step by step) for making this solution.
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 6P
Related questions
Question
can someone give me the answers please
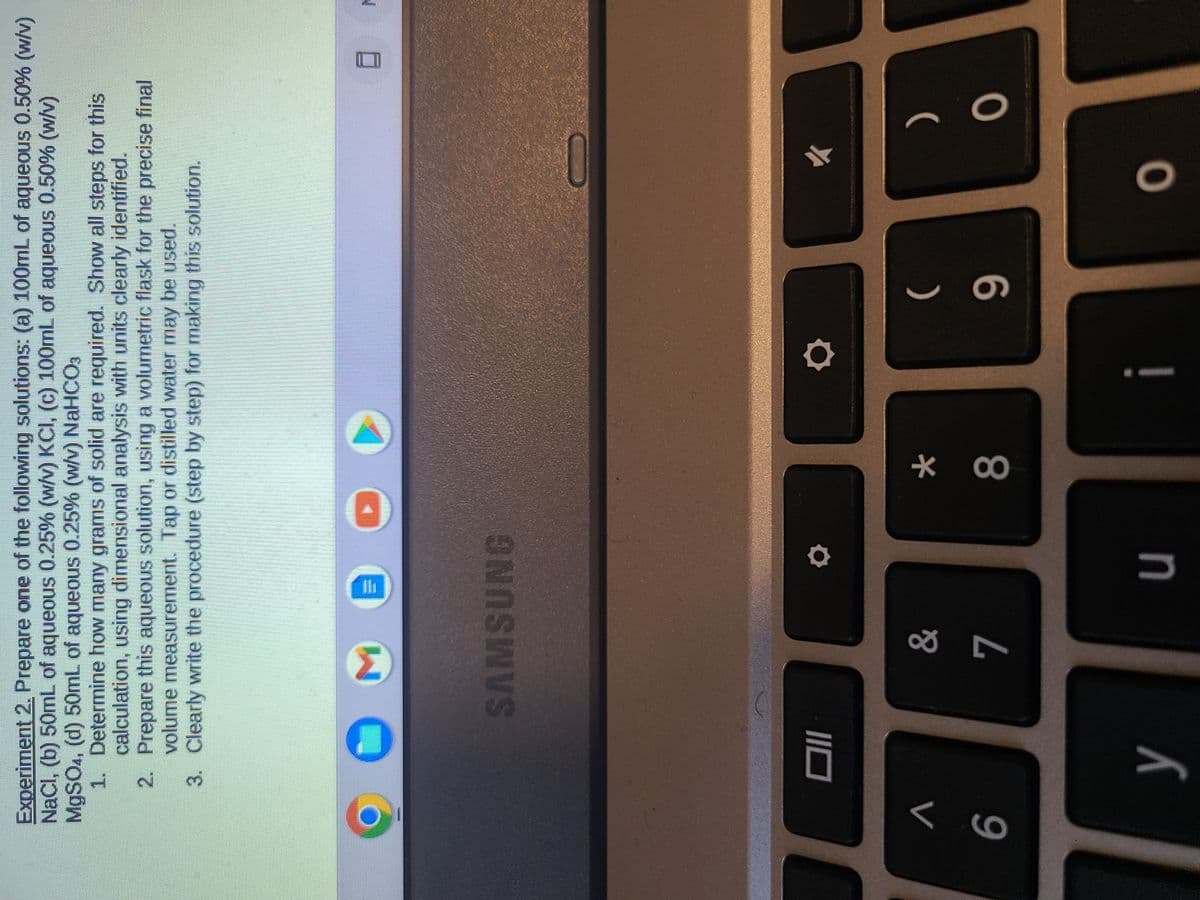
Transcribed Image Text:Experiment 2. Prepare one of the following solutions: (a) 100mL of aqueous 0.50% (w/v)
NaCl, (b) 50mL of aqueous 0.25% (w/v) KCI, (c) 100mL of aqueous 0.50% (w/v)
MgSO4, (d) 50mL of aqueous 0.25% (w/v) NaHCO3
< 6
1. Determine how many grams of solid are required. Show all steps for this
calculation, using dimensional analysis with units clearly identified.
Prepare this aqueous solution, using a volumetric flask for the precise final
volume measurement. Tap or distilled water may be used.
2.
3.
Clearly write the procedure (step by step) for making this solution.
M
SAMSUNG
&
(
7
O
y u
* 00
8
i
(
9
0
O
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

