Experiment #3- Density of Liquids and Determination of Sugar Content in Soft Drinks and Juices Report Form ples learned Ys and 1 Date Lab partners Name Type this e I Sugar Content in Knowns Generating a Calibration Curve from Density Measurements of Known Sugar Solutions Use the procedure described to determine the density of each of the known solutions, and enter your findings in the tables below. an" sectio to get you PDF file 5% Sugar Distilled Water Density of Knowns With Graduated Cylinder Mass of 10 mL graduated cylinder Volume of liquid (V) Mass of liquid + graduated cylinder Mass of liquid (m) Density of solid (D = m/V) 38.85 C6.8 45.508 t0.105 0:919 38.858 mL mL 45.12 t6.27 g/mL g/mL %3D 15% Sugar 10% Sugar With Graduated Cylinder Mass of 10 mL graduated cylinder Volume of liquid (V) Mass of liquid + graduated cylinder Mass of liquid (m) Density of solid (D = m/V) 238.788 38.81 mL 45.878 7.09 1.04 46.35-g 7,54 де g/mL 1:017 g/mL %3D are 20% Sugar With Graduated Cylinder Mass of 10 mL graduated cylinder Polume of liquid (V) lass of liquid + graduated cylinder ass of liquid (m) ensity of solid (D = m/V) ১৪ ৪7. g , এ mL 45.59.8 10.77 1.00g/mL Sugar Content of Unknowns ow the same procedure you used for the known sugar solutions to determine the density of nknown sugar solutions. Enter your measurements and findings in the tables below. Unknown #PPDe Jui ce Unknown #SPke sity of Unknowns Graduated Cylinder of 10 mL graduated cylinder ne of liquid (V) of liquid + graduated cylinder of liquid (m) w of solid (D = m/V) 38838 38.78 g 7.4 46:33 7558 1.02 mL mL 7.4 46.29 246 1.01 g/mL g/mL DENSITY OF LIQUIDS AND DETERMINATION OF SUGAR CONTENT 25 PERCENT SUGAR SAMPLE NUMBER DENSITY READING Questions to be answered for this experiment 1. To see how density, mass and volume are related, answer the following questions. a) What is the mass of 25.0 mL of olive oil having a density of 0.778 g/mL? b) What is the volume of 25.0 g of a liquid that has a density of 1.115 g/mL c) Two liquids A and B are under study by two groups of students. One group studied liquid A and found that 65.0 mL weighed 77.84 g. The other group found that 26.3 mL of liquid B weighed 31.49 g. Based on these findings, which statement is correct (use the correct number of significant figures). i) The density of liquid A is greater than that of liquid B. ii) The density of liquid A is equal to that of liquid B. iii) The density of liquid A is less than that of liquid B. aily. 2. What is the purpose of creating a calibration curve of density vs. percent sugar? 3. Attach a copy of your graph and your best fit line.
Experiment #3- Density of Liquids and Determination of Sugar Content in Soft Drinks and Juices Report Form ples learned Ys and 1 Date Lab partners Name Type this e I Sugar Content in Knowns Generating a Calibration Curve from Density Measurements of Known Sugar Solutions Use the procedure described to determine the density of each of the known solutions, and enter your findings in the tables below. an" sectio to get you PDF file 5% Sugar Distilled Water Density of Knowns With Graduated Cylinder Mass of 10 mL graduated cylinder Volume of liquid (V) Mass of liquid + graduated cylinder Mass of liquid (m) Density of solid (D = m/V) 38.85 C6.8 45.508 t0.105 0:919 38.858 mL mL 45.12 t6.27 g/mL g/mL %3D 15% Sugar 10% Sugar With Graduated Cylinder Mass of 10 mL graduated cylinder Volume of liquid (V) Mass of liquid + graduated cylinder Mass of liquid (m) Density of solid (D = m/V) 238.788 38.81 mL 45.878 7.09 1.04 46.35-g 7,54 де g/mL 1:017 g/mL %3D are 20% Sugar With Graduated Cylinder Mass of 10 mL graduated cylinder Polume of liquid (V) lass of liquid + graduated cylinder ass of liquid (m) ensity of solid (D = m/V) ১৪ ৪7. g , এ mL 45.59.8 10.77 1.00g/mL Sugar Content of Unknowns ow the same procedure you used for the known sugar solutions to determine the density of nknown sugar solutions. Enter your measurements and findings in the tables below. Unknown #PPDe Jui ce Unknown #SPke sity of Unknowns Graduated Cylinder of 10 mL graduated cylinder ne of liquid (V) of liquid + graduated cylinder of liquid (m) w of solid (D = m/V) 38838 38.78 g 7.4 46:33 7558 1.02 mL mL 7.4 46.29 246 1.01 g/mL g/mL DENSITY OF LIQUIDS AND DETERMINATION OF SUGAR CONTENT 25 PERCENT SUGAR SAMPLE NUMBER DENSITY READING Questions to be answered for this experiment 1. To see how density, mass and volume are related, answer the following questions. a) What is the mass of 25.0 mL of olive oil having a density of 0.778 g/mL? b) What is the volume of 25.0 g of a liquid that has a density of 1.115 g/mL c) Two liquids A and B are under study by two groups of students. One group studied liquid A and found that 65.0 mL weighed 77.84 g. The other group found that 26.3 mL of liquid B weighed 31.49 g. Based on these findings, which statement is correct (use the correct number of significant figures). i) The density of liquid A is greater than that of liquid B. ii) The density of liquid A is equal to that of liquid B. iii) The density of liquid A is less than that of liquid B. aily. 2. What is the purpose of creating a calibration curve of density vs. percent sugar? 3. Attach a copy of your graph and your best fit line.
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
100%
The first pic are my results the 2nd one is the question
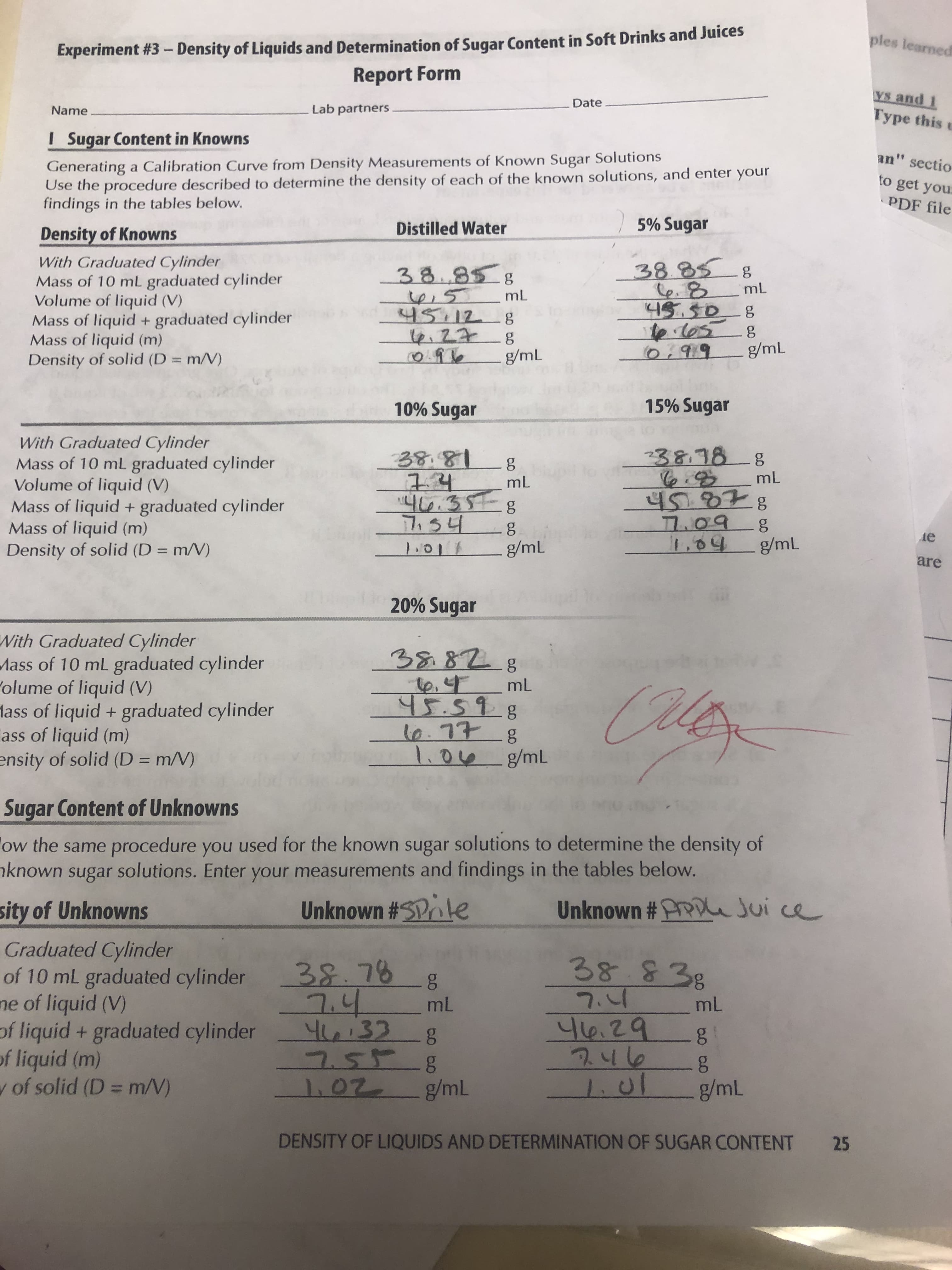
Transcribed Image Text:Experiment #3- Density of Liquids and Determination of Sugar Content in Soft Drinks and Juices
Report Form
ples learned
Ys and 1
Date
Lab partners
Name
Type this e
I Sugar Content in Knowns
Generating a Calibration Curve from Density Measurements of Known Sugar Solutions
Use the procedure described to determine the density of each of the known solutions, and enter your
findings in the tables below.
an" sectio
to get you
PDF file
5% Sugar
Distilled Water
Density of Knowns
With Graduated Cylinder
Mass of 10 mL graduated cylinder
Volume of liquid (V)
Mass of liquid + graduated cylinder
Mass of liquid (m)
Density of solid (D = m/V)
38.85
C6.8
45.508
t0.105
0:919
38.858
mL
mL
45.12
t6.27
g/mL
g/mL
%3D
15% Sugar
10% Sugar
With Graduated Cylinder
Mass of 10 mL graduated cylinder
Volume of liquid (V)
Mass of liquid + graduated cylinder
Mass of liquid (m)
Density of solid (D = m/V)
238.788
38.81
mL
45.878
7.09
1.04
46.35-g
7,54
де
g/mL
1:017
g/mL
%3D
are
20% Sugar
With Graduated Cylinder
Mass of 10 mL graduated cylinder
Polume of liquid (V)
lass of liquid + graduated cylinder
ass of liquid (m)
ensity of solid (D = m/V)
১৪ ৪7. g
, এ
mL
45.59.8
10.77
1.00g/mL
Sugar Content of Unknowns
ow the same procedure you used for the known sugar solutions to determine the density of
nknown sugar solutions. Enter your measurements and findings in the tables below.
Unknown #PPDe Jui ce
Unknown #SPke
sity of Unknowns
Graduated Cylinder
of 10 mL graduated cylinder
ne of liquid (V)
of liquid + graduated cylinder
of liquid (m)
w of solid (D = m/V)
38838
38.78 g
7.4
46:33
7558
1.02
mL
mL
7.4
46.29
246
1.01
g/mL
g/mL
DENSITY OF LIQUIDS AND DETERMINATION OF SUGAR CONTENT
25

Transcribed Image Text:PERCENT SUGAR
SAMPLE NUMBER
DENSITY READING
Questions to be answered for this experiment
1. To see how density, mass and volume are related, answer the following questions.
a) What is the mass of 25.0 mL of olive oil having a density of 0.778 g/mL?
b) What is the volume of 25.0 g of a liquid that has a density of 1.115 g/mL
c) Two liquids A and B are under study by two groups of students. One group studied liquid A
and found that 65.0 mL weighed 77.84 g. The other group found that 26.3 mL of liquid B
weighed 31.49 g. Based on these findings, which statement is correct (use the correct
number of significant figures).
i) The density of liquid A is greater than that of liquid B.
ii) The density of liquid A is equal to that of liquid B.
iii) The density of liquid A is less than that of liquid B.
aily.
2. What is the purpose of creating a calibration curve of density vs. percent sugar?
3. Attach a copy of your graph and your best fit line.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning