experiment A pair of 100 mL samples of water are taken from a well bored into a large underground salt (NaCl) deposit. Sample #1 is from the top of the well, and is initially at 32 °C. Sample #2 is from a depth of 50. m, and is initially at 42 °C. Both samples are allowed to come to room temperature (20. °C) and 1 atm pressure. An NaCl precipitate is seen to form in Sample #1. Two 250 mL samples of water are drawn from a deep well bored into a large underground salt (NaCl) deposit. Sample #1 is from the top of the well, and is initially at 42 °C. Sample #2 is from a depth of 150 m, and is initially at 8 °C. Both samples are allowed to come to room temperature (20 °C) and 1 atm pressure. An NaCl precipitate is seen to form in Sample #1. predicted observation (choose one) A bigger mass of NaCl precipitate will form in Sample #2. A smaller mass of NaCl precipitate will form in Sample #2. The same mass of NaCl precipitate will form in Sample #2. O No precipitate will form in Sample #2. I need more information to predict whether and how much precipitate will form in Sample #2. A bigger mass of NaCl precipitate will form in Sample #2. A smaller mass of NaCl precipitate will form in Sample #2. The same mass of NaCl precipitate will form in Sample #2. No precipitate will form in Sample #2. I need more information to predict whether and how much precipitate will form in Sample #2.
experiment A pair of 100 mL samples of water are taken from a well bored into a large underground salt (NaCl) deposit. Sample #1 is from the top of the well, and is initially at 32 °C. Sample #2 is from a depth of 50. m, and is initially at 42 °C. Both samples are allowed to come to room temperature (20. °C) and 1 atm pressure. An NaCl precipitate is seen to form in Sample #1. Two 250 mL samples of water are drawn from a deep well bored into a large underground salt (NaCl) deposit. Sample #1 is from the top of the well, and is initially at 42 °C. Sample #2 is from a depth of 150 m, and is initially at 8 °C. Both samples are allowed to come to room temperature (20 °C) and 1 atm pressure. An NaCl precipitate is seen to form in Sample #1. predicted observation (choose one) A bigger mass of NaCl precipitate will form in Sample #2. A smaller mass of NaCl precipitate will form in Sample #2. The same mass of NaCl precipitate will form in Sample #2. O No precipitate will form in Sample #2. I need more information to predict whether and how much precipitate will form in Sample #2. A bigger mass of NaCl precipitate will form in Sample #2. A smaller mass of NaCl precipitate will form in Sample #2. The same mass of NaCl precipitate will form in Sample #2. No precipitate will form in Sample #2. I need more information to predict whether and how much precipitate will form in Sample #2.
Chapter2: Crystallization
Section: Chapter Questions
Problem 3Q
Related questions
Question
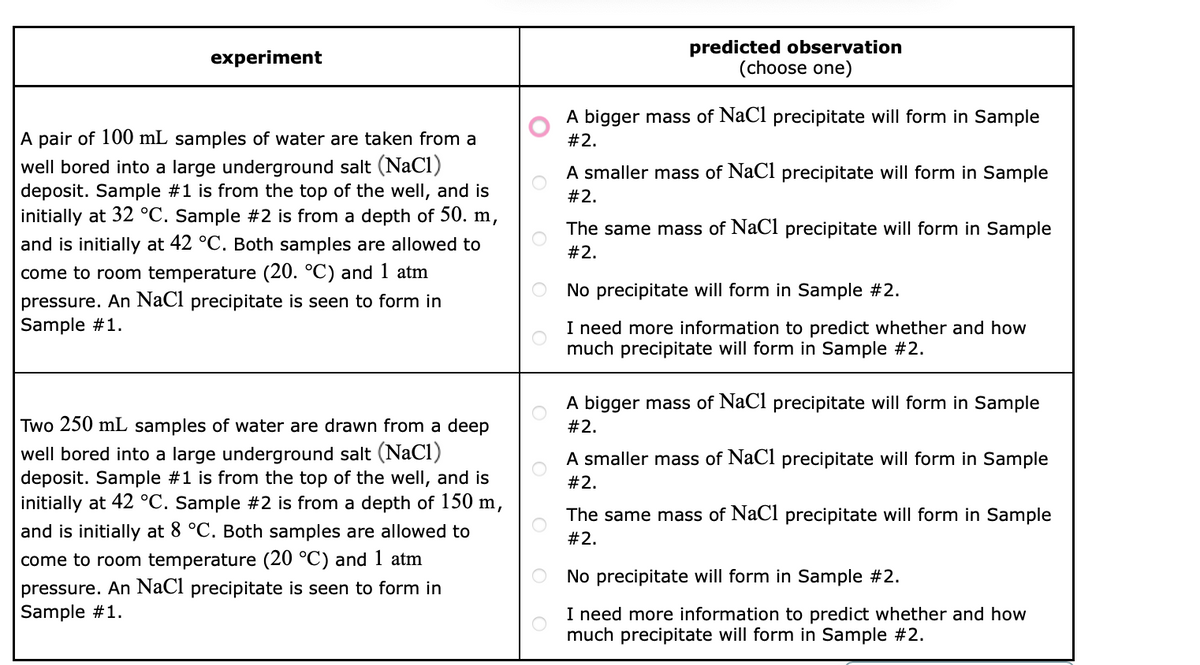
Transcribed Image Text:experiment
A pair of 100 mL samples of water are taken from a
well bored into a large underground salt (NaCl)
deposit. Sample #1 is from the top of the well, and is
initially at 32 °C. Sample #2 is from a depth of 50. m,
and is initially at 42 °C. Both samples are allowed to
come to room temperature (20. °C) and 1 atm
pressure. An NaCl precipitate is seen to form in
Sample #1.
Two 250 mL samples of water are drawn from a deep
well bored into a large underground salt (NaCl)
deposit. Sample #1 is from the top of the well, and is
initially at 42 °C. Sample #2 is from a depth of 150 m,
and is initially at 8 °C. Both samples are allowed to
come to room temperature (20 °C) and 1 atm
pressure. An NaCl precipitate is seen to form in
Sample #1.
predicted observation
(choose one)
A bigger mass of NaCl precipitate will form in Sample
#2.
A smaller mass of NaCl precipitate will form in Sample
#2.
The same mass of NaCl precipitate will form in Sample
#2.
No precipitate will form in Sample #2.
I need more information to predict whether and how
much precipitate will form in Sample #2.
A bigger mass of NaCl precipitate will form in Sample
#2.
A smaller mass of NaCl precipitate will form in Sample
#2.
The same mass of NaCl precipitate will form in Sample
#2.
No precipitate will form in Sample #2.
I need more information to predict whether and how
much precipitate will form in Sample #2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT