Explain why the transition metals in periods 5 and 8 have nearly identical radii in each group. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. electron shell build-up 4f decrease larger Zett number of electrons nuclear build-up 4d increase 1. We expect radii in a group to number increases. 2. However, the trend. moving down the periodic table as principle quantum associated with filling of the Reset Help subshell counteracts this 3. The increased causes the radil of the metals in group 6 to be expected, and period 5 and 6 metals in the same group to have very similar radii. than
Explain why the transition metals in periods 5 and 8 have nearly identical radii in each group. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. electron shell build-up 4f decrease larger Zett number of electrons nuclear build-up 4d increase 1. We expect radii in a group to number increases. 2. However, the trend. moving down the periodic table as principle quantum associated with filling of the Reset Help subshell counteracts this 3. The increased causes the radil of the metals in group 6 to be expected, and period 5 and 6 metals in the same group to have very similar radii. than
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter22: The Transition Elements And Coordination Compounds
Section: Chapter Questions
Problem 22.31QP
Related questions
Question
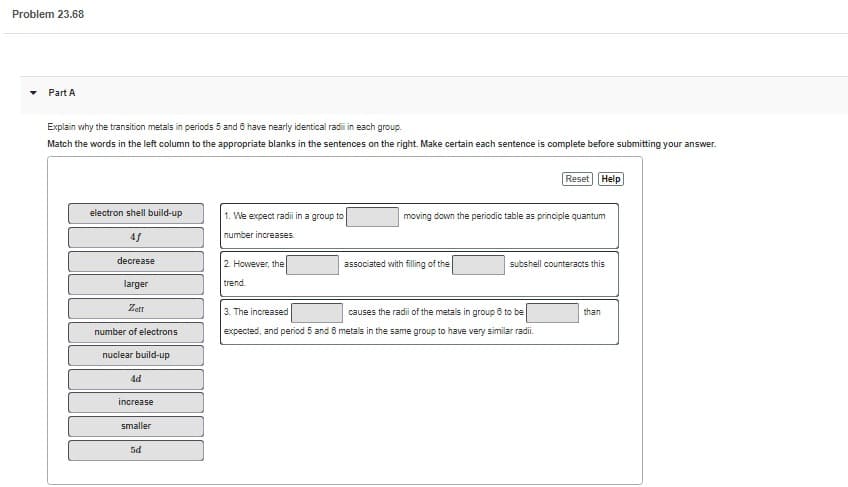
Transcribed Image Text:Problem 23.68
Part A
Explain why the transition metals in periods 5 and 6 have nearly identical radili in each group.
Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.
electron shell build-up
4f
decrease
larger
Zert
number of electrons
nuclear build-up
4d
increase
smaller
5d
1. We expect radii in a group to
number increases.
2. However, the
trend.
moving down the periodic table as principle quantum
associated with filling of the
Reset Help
subshell counteracts this
3. The increased
causes the radii of the metals in group 6 to be
expected, and period 5 and 6 metals in the same group to have very similar radii.
than
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning