Chapter17: Alcohols And Phenols
Section17.SE: Something Extra
Problem 67AP: Dehydration of trans-2-methylcyclopentanol with POCl3 in pyridine yields predominantly...
Related questions
Question
I need a break down and explnation of the mechanism step by step
Transcribed Image Text:9 H® From water
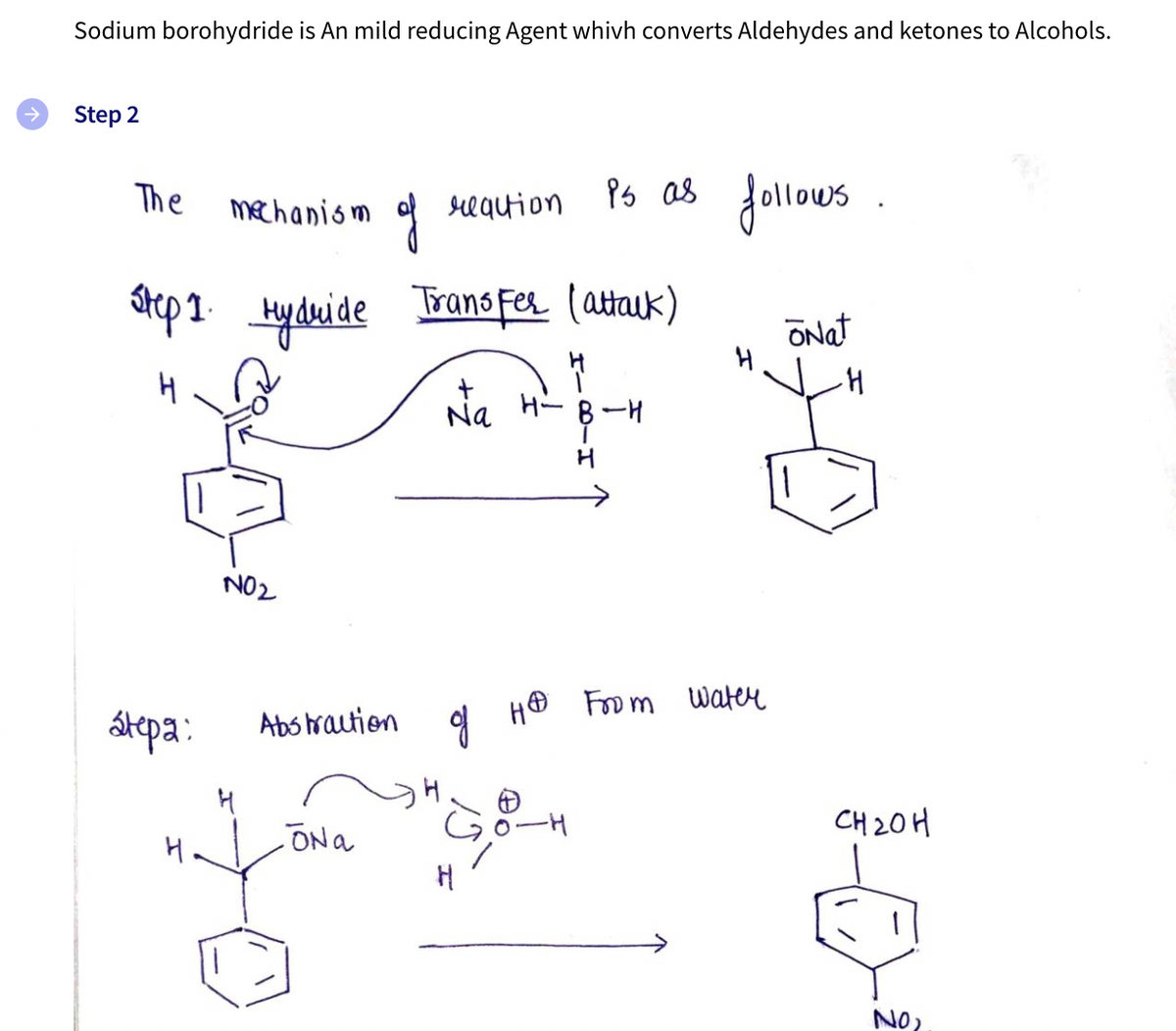
Sodium borohydride is An mild reducing Agent whivh converts Aldehydes and ketones to Alcohols.
Step 2
Ps as .
Jollous
The
mechanism
requion
atep1 Hyduide
Trans Fer (attauk)
ONat
Na
H- B-H
H
->
NO2
átepa:
Abs hraution
CH 20H
H.
ONa
NO?
130
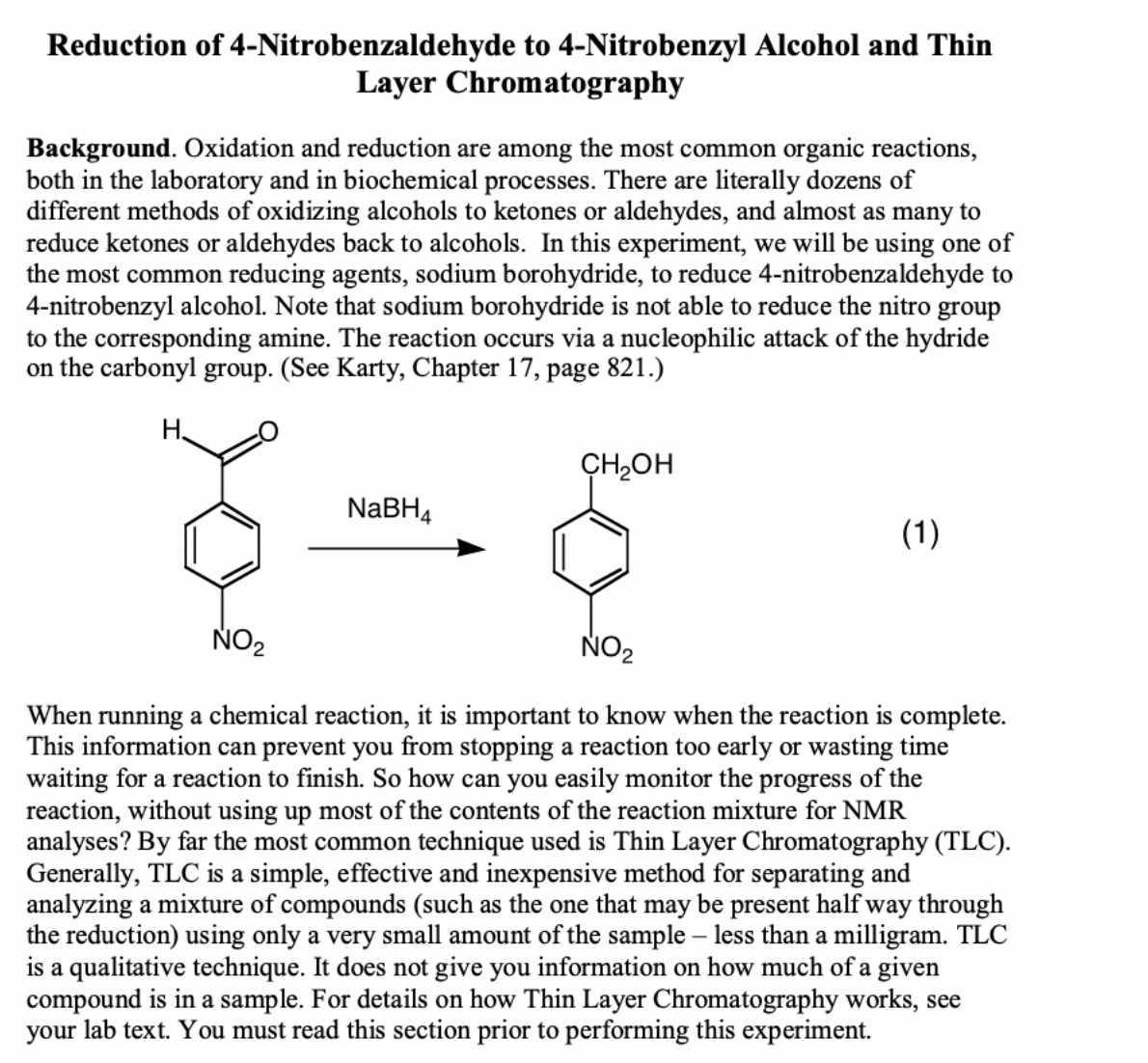
Transcribed Image Text:Reduction of 4-Nitrobenzaldehyde to 4-Nitrobenzyl Alcohol and Thin
Layer Chromatography
Background. Oxidation and reduction are among the most common organic reactions,
both in the laboratory and in biochemical processes. There are literally dozens of
different methods of oxidizing alcohols to ketones or aldehydes, and almost as many to
reduce ketones or aldehydes back to alcohols. In this experiment, we will be using one of
the most common reducing agents, sodium borohydride, to reduce 4-nitrobenzaldehyde to
4-nitrobenzyl alcohol. Note that sodium borohydride is not able to reduce the nitro group
to the corresponding amine. The reaction occurs via a nucleophilic attack of the hydride
on the carbonyl group. (See Karty, Chapter 17, page 821.)
H.
CH2OH
NaBH4
(1)
NO2
When running a chemical reaction, it is important to know when the reaction is complete.
This information can prevent you from stopping a reaction too early or wasting time
waiting for a reaction to finish. So how can you easily monitor the progress of the
reaction, without using up most of the contents of the reaction mixture for NMR
analyses? By far the most common technique used is Thin Layer Chromatography (TLC).
Generally, TLC is a simple, effective and inexpensive method for separating and
analyzing a mixture of compounds (such as the one that may be present half way through
the reduction) using only a very small amount of the sample – less than a milligram. TLC
is a qualitative technique. It does not give you information on how much of a given
compound is in a sample. For details on how Thin Layer Chromatography works, see
your lab text. You must read this section prior to performing this experiment.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT