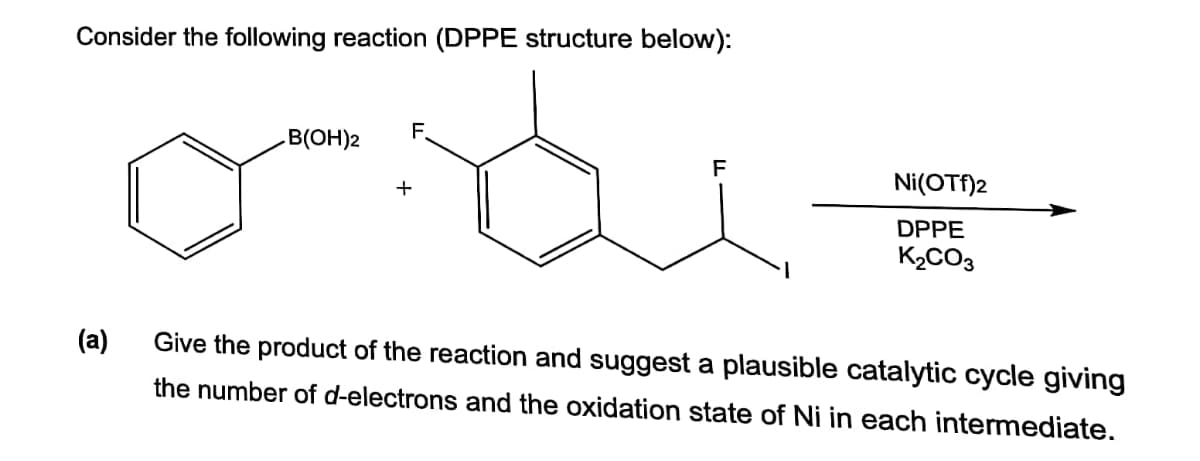
F. B(OH)2 Ni(OTf)2 DPPE K2CO3 (a) Give the product of the reaction and suggest a plausible catalytic cycle giving the number of d-electrons and the oxidation state of Ni in each intermediate.
Q: In a three-electrode cell, the [Select] is used to measure the potential of the working electrode…
A: Refrence Electrode - The ultimate purpose of Reference Electrode is used to provide a stable…
Q: Heliox is a helium-oxygen mixture that may be used in scuba tanks for divers working at great…
A: Given: Volume of helium gas = 6.25 L Pressure of helium gas = 1.260 × 103 psi Volume of oxygen gas =…
Q: A solution with 5 mg/mL of a colored protein has an absorbance of 0.250. What is the concentration…
A:
Q: How many minutes will it take to plate out 4.56 g of Ni metal from a solution of Ni2+ using a…
A: Given: Mass of Ni deposited = 4.56 g Current passed = 45.5 A Molar mass of Ni = 58.69 g/mol
Q: Q2 :- Calculate the percentage of p in the sample of a 0.703 g weight, produced 0.223 g of Mg.P:O7…
A:
Q: Encircle the chiral center/s in each molecule. Assign R or S configurations for each chiral center.…
A: According to CIP rule in terms of R/S designation , clock wise rotation with lower priority group in…
Q: What is molar mass of oxygen
A:
Q: Water with an alkalinity of 2.00 x 10-3 eq/L has a pH of 7.0. Calculate [CO2], [HCO3-], [CO32-], and…
A:
Q: Phosphate in wastewater samples can be precipitated out as Ca10(PO4)6(OH)2 (1,004.6 g/mol).…
A: Given that phosphate in wastewater samples can be precipitated out as Ca10PO46OH2.And municipal…
Q: In voltaic cells, the purpose of the salt bridge ---- O is tightly plugged with firm agar cell to…
A: Voltaic cell are the cells which converts chemical energy into electrical energy.
Q: Calculate the molarity of a solution by dissolving 15g of sugar C12H22O11 in 3.50×10²mL water…
A: The molarity of a solution is the ratio between the number of moles of solute to the solution…
Q: Question 5 Provide the oxidation number (with correct sign) for the underlined element in the…
A: Entropy of a system can be defined as the degree of disorder present in the system. it is denoted…
Q: Suppose a tank of oxygen-enriched air prepared for scuba diving has a volume of 11.04 L and a…
A: Partial pressure of oxygen can be used to calculate the number of moles of oxygen present in the…
Q: Calculate E for a lead acid battery with a sulfuric acid concentration of 2.00 M. PbO2(s) +…
A: The given lead acid battery is PbO2(s) + Pb(s) + 2 H2SO4(aq) →2 PbSO4(s) + 2 H2O(l) Eo = +…
Q: What are the possible sources of error based from how the researcher obtained the water samples from…
A: A question based on tools in analytical chemistry that is to be accomplished.
Q: Ag wire used to measure the concentration of CI ion is an example of a what type of electrode? O…
A:
Q: 1. Use structures and words to show how the cyclization of the linear glucose molecule to the cyclic…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Calculate the mole fraction and the molality of 65.0g NaCl in 300g H2O
A:
Q: Which aqueous solution has the lowest pH? O a. 0.30 M HCI O b.0.30 M NH 3 Oc.0.30 M CH 3COOH O…
A:
Q: A 0.7785 g sample containing hydrazine (N2H4, rocket fuel) was dissolved and diluted to 250.0 mL in…
A: Answer: This question is based on stoichiometric calculation where we have to convert the moles of…
Q: It was found that 3.85 g of a compound of phosphorus and chlorine contained 0.165 g of phosphorus.…
A:
Q: Question 2 At 25 Ca solution has a hydroxide-ion concentration of 7.47 x 10 M. What is its…
A: You have asked multiple type questions, we will solve only first questions for you. If you want any…
Q: Which of the statements below is true for a reaction that is endothermic and leads to an decrease in…
A:
Q: The hydrolysis of a substrate, S, by an enzyme has been studied in the lab. The following initial…
A: The data given is, Given: [S] = 1.1 × 10-4 M
Q: Which element has the electron configuration 1s²2s²2p⁶3s²3p⁶4s²3d⁵?
A: Total number of electrons =( 2+2+6+2+6+2+5 ) = 25 We know that, Atomic number = number of protons…
Q: (1R)-1-chloro-1-cyclohexylpropan-1-ol Draw the bond-line structure of the said compound
A:
Q: Which type of interaction from the diagram must be strong in order to dissolve a salt? When…
A: Solution is made up of solute and solvent .Solute is present in small amount and solvent is present…
Q: What Concentration of sulfite ion will produce a solution with a ph of 10.00?
A: pH of any solution is called as the concentration of the H+ ions present in it. The pH of the…
Q: Write the reaction quotient, Qc, for each of the following reactions: (a) The first step in nitric…
A: Given reaction is : NH3 (g) + O2 (g) ---------> NO (g) + H2O (g) Write the expression of…
Q: Predict the major product(s) obtained the following compound undergoes hydrolysis in the presence of…
A: Note : Since you have posted multiple questions, we are entitled to answer the first only. Please…
Q: What is the sign of Δ S for the reaction below as written? N2O4(g) ---------> N2(g) + 2 O2(g)…
A: Answer: Entropy is the measure of disorderliness or randomness of the system. In more possible…
Q: Current Attempt in Progress What is the mass defect for americium-241 if the mass of the nucleus is…
A:
Q: 4. A. With specific examples, discuss two practical applications of Galvanic cell and two practical…
A: 1) A battery, which is actually an electric cell, is a device that produces electricity from a…
Q: Please show complete mechanism with all steps with naming OH OH H2, Pd/C HO Меон, EtOH 23 °C, 100%…
A: Note : Benzyl deprotection using hydrogenation. ( For mechanism see below).
Q: -cuttim-which cation is a weakacd2 Na Mm4+ giveanewer. with explanation Than k your
A: This question is related to acid-base concept. An acid is a species which can accept a lone pair of…
Q: Which two functional groups are in the molecule "The left shown functional group below? Use is a…
A: Organic chemistry is branch of chemistry in which organic reactant react to form organic products.
Q: CO(g) + Cl2(g) ⇌ COCl2(g) Suppose a system at equilibrium contains 102 atm CO, 229 atm Cl2, and…
A: Answer: This question is based on the concept that if we add reactant in the system which is at…
Q: CH, d 2, CH CH CH 7-tert-butyl-8-isobutyl-4-isopropyldodecane (hyphenated words aren't…
A: IUPAC nomenclature is used for naming the organic compound as recommended by international union of…
Q: Give the maximum number of orbitals in an atom that can have these quantum numbers: n = 3, ml = -1
A: In this question, we will give the maximum number of orbitals in an atom when quantum number n = 3…
Q: 1) Nal Br a) 2) NaCN, acetone I) NAOC(CH,), heat b) Br 2) BH, THF; 3) H,O, NaOH(aq) 1) HCI c) 2)…
A: Since you have posted a question with multiple sub-parts, we will solve the first three subparts for…
Q: Using the half reaction method, balance the redox reaction under basic conditions: Zn(S) + NO3-(aq)…
A:
Q: :0: CH3OH2* protonation H3C CH3OH nucleophilic addition CH3 CH3OH H Drawing :OCH, deprotonatio =D1…
A:
Q: The pH of a 0.24M solution of pentanoic acid (HC, H,0,) is measured to be 2.73. Calculate the acid…
A:
Q: ow many moles of UF6 would have to be decom rbon is available.) i mol UF6
A: The balanced chemical reaction, 3C + 2UF6 ---> 3CF4 + 2U 3 mol CF4 required = 2 mol UF6 2.06 mol…
Q: Given the following information about an enzymatic reaction, calculate the initial velocity. Your…
A: Given,Vmax = 5 nM sec-1KM = 1 mMS = 10 mMRequired, The initial velocity of an…
Q: Soap production I would like to produce a soap in the lab. I take 100 g of oil/fat and now I would…
A: Given that - Mass of oil/fat like tripalmitin, which is commonly used in preparation of soap = 100…
Q: Determine the major product of the given reaction below by drawing the complete reaction mechanism.…
A: Detail mechanistic pathway is given below
Q: Ammonia reacts with O2 to form either NO(g) or NO2 (g) according to the following UNBALANCED…
A:
Q: 6 H20) + 6 CO2)- C6H1206(s) + 6 O2), AH-+2803 kl/mol. In order to form 78.155 g of C6H1206 (molar…
A:
Q: 7) The o-complex formed for the bromination of benzoic acid should be Br. H. .COOH .COOH СООН Br…
A:
Step by step
Solved in 2 steps with 2 images

- Give out methods of preparation for cyclobutadienee complexsDescribe the species that would result from the subsequent transfer of the proton from the metal to one of the Cp rings of ferrocene. Give the formal oxidation state of the metal centre and the valence electron countWrite all the isomers of [PtCl(CO)(PMe3)(C2H4)]+ and use the trans directing effect of ligands to suggest the reaction pathways to synthesize the above isomers from [PtCl4] 2-
- Butan-1-ol can be oxidised by acidified potassium dichromate( VI) using two different methods. (a) In the first method, butan-1-ol is added dropwise to acidified potassium dichromate( VI) and the product is distilled off immediately. (i) Using the symbol [O] for the oxidising agent, write an equation for this oxidation of butan-1-ol, showing clearly the structure of the product.State what colour change you would observe. Equation ........................................................................................................... Colour change .................................................................................................. (ii) Butan-1-ol and butan-2-ol give different products on oxidation by this first method. By stating a reagent and the observation with each compound, give a simple test to distinguish between these two oxidation products. Reagent…Describe and map the reaction between Potassium dichromate (K2Cr2O7) and Sodium thiosulfate as a pentahydrate (Na2S2O3·5H2O). Show all products.Name the reaction and show the mechanism including the formation of show formation of an Si-F bond in the cleavage of the silyl group.
- One of the steps in fat metabolism is the hydration of crotonate to yield 3-hydroxybutyrate. This reaction occurs by addition of —OH to the Si face at C3, followed by protonation at C2, also from the Si face. Draw the product of the reaction, showing the stereochemistry of each step.For each of the following mixtures of reactants, give (i) a plausible chemical equation and (ii) structurefor the organometallic product, and (iii) general reason for the course of the reaction: (a)methyllithium and W(CO)6, (b) Co2(CO)8 and AlBr3.In an experiment, triphenylmethanol is prepared using the Grignard reaction. Reaction of bromobenzene with magnesium in ether produces phenylmagnesium bromide. This Grignard reagent then reacts with methyl benzoate to produce the corresponding alkoxide. Reaction of the alkoxide with aqueous acid then produces the alcohol. Give a plausible, three dimensional structure for the complex RMgBr-2(C2H5)2O. How do you think the ether molecules are bonded to the Grignard reagent?
- The following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Questions: (i) Give the name and suggest the colour of the precipitate B. Hence explain why it is necessary to recrystallize B several times. (ii) Why was it necessary to obtain a constant melting point for B?The following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. 1g of phenylamine yielded 1.2g of compound A. Calculate the percentage yield of the reaction.The following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Why was it necessary to obtain a constant melting point for B?

