FIGURE 10.31 The energy diagram for molecular oxygen, O2. Energy (×10-1º J) 20 16 12 8 PE 4 x (nm) 0.0 0.1 0.2 0.3 0.4
FIGURE 10.31 The energy diagram for molecular oxygen, O2. Energy (×10-1º J) 20 16 12 8 PE 4 x (nm) 0.0 0.1 0.2 0.3 0.4
Related questions
Question
An energy diagram for molecular oxygen, O2, is shown. A germicidal lamp for sterilizing equipment uses short-wavelength ultraviolet radiation at 185 nm. At this wavelength, each photon, or quantum, of ultraviolet light has 10.7 x 10-19 J of energy. If a molecule of O2 at room temperature absorbs one photon of light from the lamp, does this provide enough energy to split the molecule? If so, what will be the kinetic energy of the atoms after they have separated?
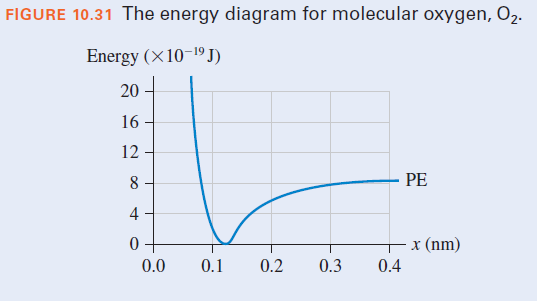
Transcribed Image Text:FIGURE 10.31 The energy diagram for molecular oxygen, O2.
Energy (×10-1º J)
20
16
12
8
PE
4
x (nm)
0.0
0.1
0.2
0.3
0.4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images
