Fill in the blanks to complete the balanced formation reaction for the following compound: Notes for filling blanks: • Enter one reactant per blank, in alphabetical order of the element symbols (such as C, CI, H, N, O, etc.) • Include stoichiometric coefficients with no space before the element (such as "2N2 (g)"); coefficients of "1" may be omitted; fractional coefficients may be written in parentheses or as decimal values (such as "(1/2)N2 (g)" or "0.5N2 (g)") • Include subscript numbers as standard text (such as "N2 (g)") • Phases must be included, with a space between the element and phase (such as "N2 (g)") • Incorrect capitalization of elements will be marked as incorrect Formation reaction of NH3 (1) (ammonia): → NH3 (1)
Fill in the blanks to complete the balanced formation reaction for the following compound: Notes for filling blanks: • Enter one reactant per blank, in alphabetical order of the element symbols (such as C, CI, H, N, O, etc.) • Include stoichiometric coefficients with no space before the element (such as "2N2 (g)"); coefficients of "1" may be omitted; fractional coefficients may be written in parentheses or as decimal values (such as "(1/2)N2 (g)" or "0.5N2 (g)") • Include subscript numbers as standard text (such as "N2 (g)") • Phases must be included, with a space between the element and phase (such as "N2 (g)") • Incorrect capitalization of elements will be marked as incorrect Formation reaction of NH3 (1) (ammonia): → NH3 (1)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.69PAE: 4.69 The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to...
Related questions
Question
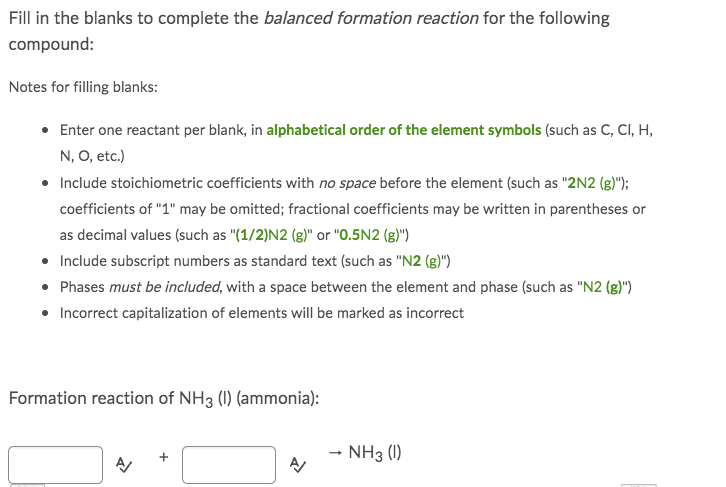
Transcribed Image Text:Fill in the blanks to complete the balanced formation reaction for the following
compound:
Notes for filling blanks:
• Enter one reactant per blank, in alphabetical order of the element symbols (such as C, CI, H,
N, O, etc.)
• Include stoichiometric coefficients with no space before the element (such as "2N2 (g)");
coefficients of "1" may be omitted; fractional coefficients may be written in parentheses or
as decimal values (such as "(1/2)N2 (g)" or "0.5N2 (g)")
• Include subscript numbers as standard text (such as "N2 (g)")
• Phases must be included, with a space between the element and phase (such as "N2 (g)")
• Incorrect capitalization of elements will be marked as incorrect
Formation reaction of NH3 (1) (ammonia):
→ NH3 (1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning