Fill out this table. Once that is done, predict the order of the boiling points from lowest to the highest. Substance ethanol 1-butanol 1-propanol C3H7OH n-pentane methanol Formula n-hexane C₂H5OH C4H,OH C5H12 CH3OH C6H14 H₂C Structural Formulas CH₂ LOH Molecular Weight Hydrogen Bond (Yes or No)
Fill out this table. Once that is done, predict the order of the boiling points from lowest to the highest. Substance ethanol 1-butanol 1-propanol C3H7OH n-pentane methanol Formula n-hexane C₂H5OH C4H,OH C5H12 CH3OH C6H14 H₂C Structural Formulas CH₂ LOH Molecular Weight Hydrogen Bond (Yes or No)
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.68P
Related questions
Question
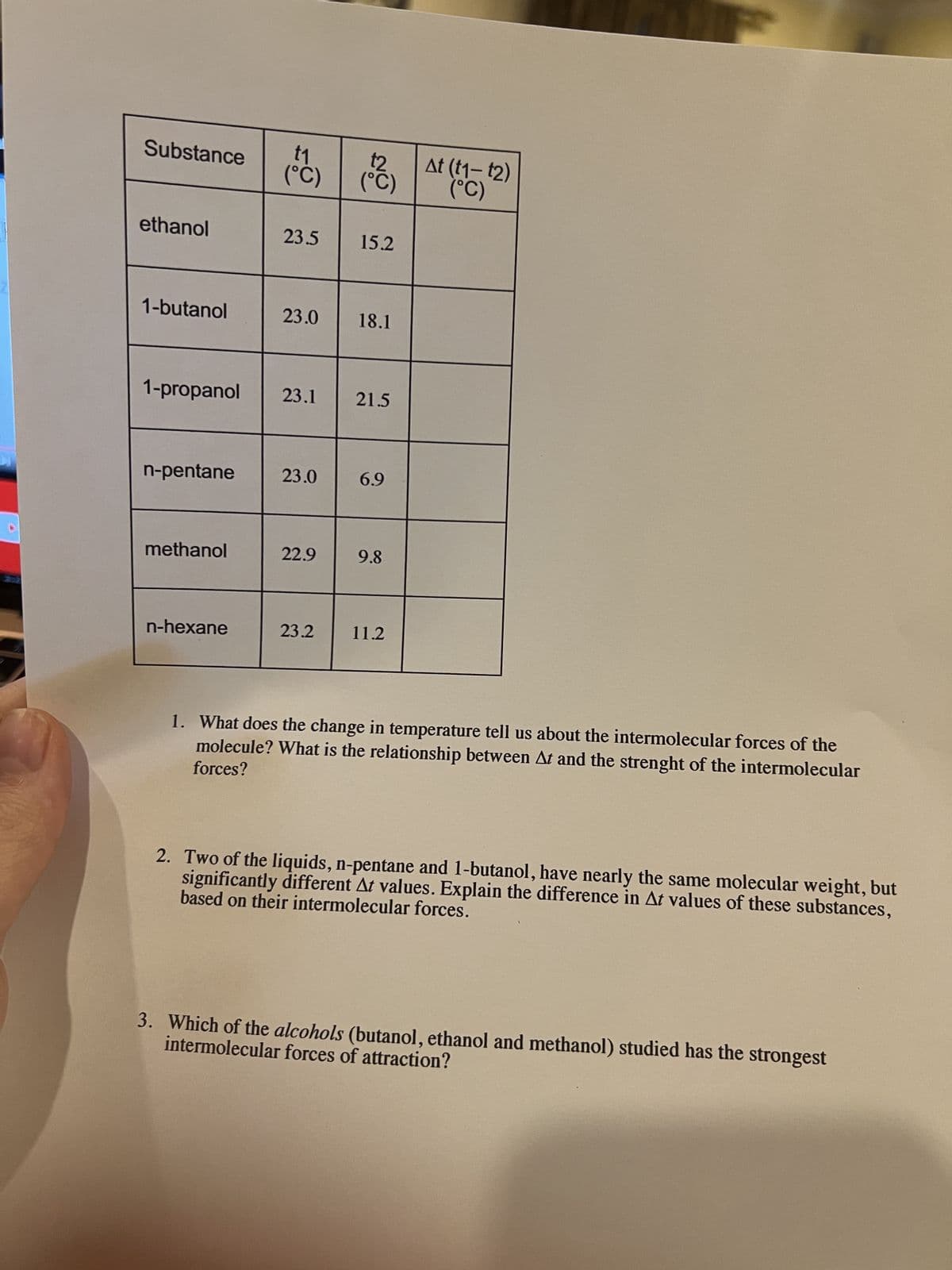
Transcribed Image Text:4
t₁
Substance (C)
ethanol
1-butanol
1-propanol
n-pentane
methanol
n-hexane
23.5
23.1
23.0 18.1
23.0
22.9
(°C)
23.2
15.2
21.5
6.9
9.8
11.2
At (t1- t2)
(°C)
1. What does the change in temperature tell us about the intermolecular forces of the
forces?
molecule? What is the relationship between At and the strenght of the intermolecular
2. Two of the liquids, n-pentane and 1-butanol, have nearly the same molecular weight, but
based on their intermolecular forces.
significantly different At values. Explain the difference in At values of these substances,
intermolecular forces of attraction?
3. Which of the alcohols (butanol, ethanol and methanol) studied has the strongest
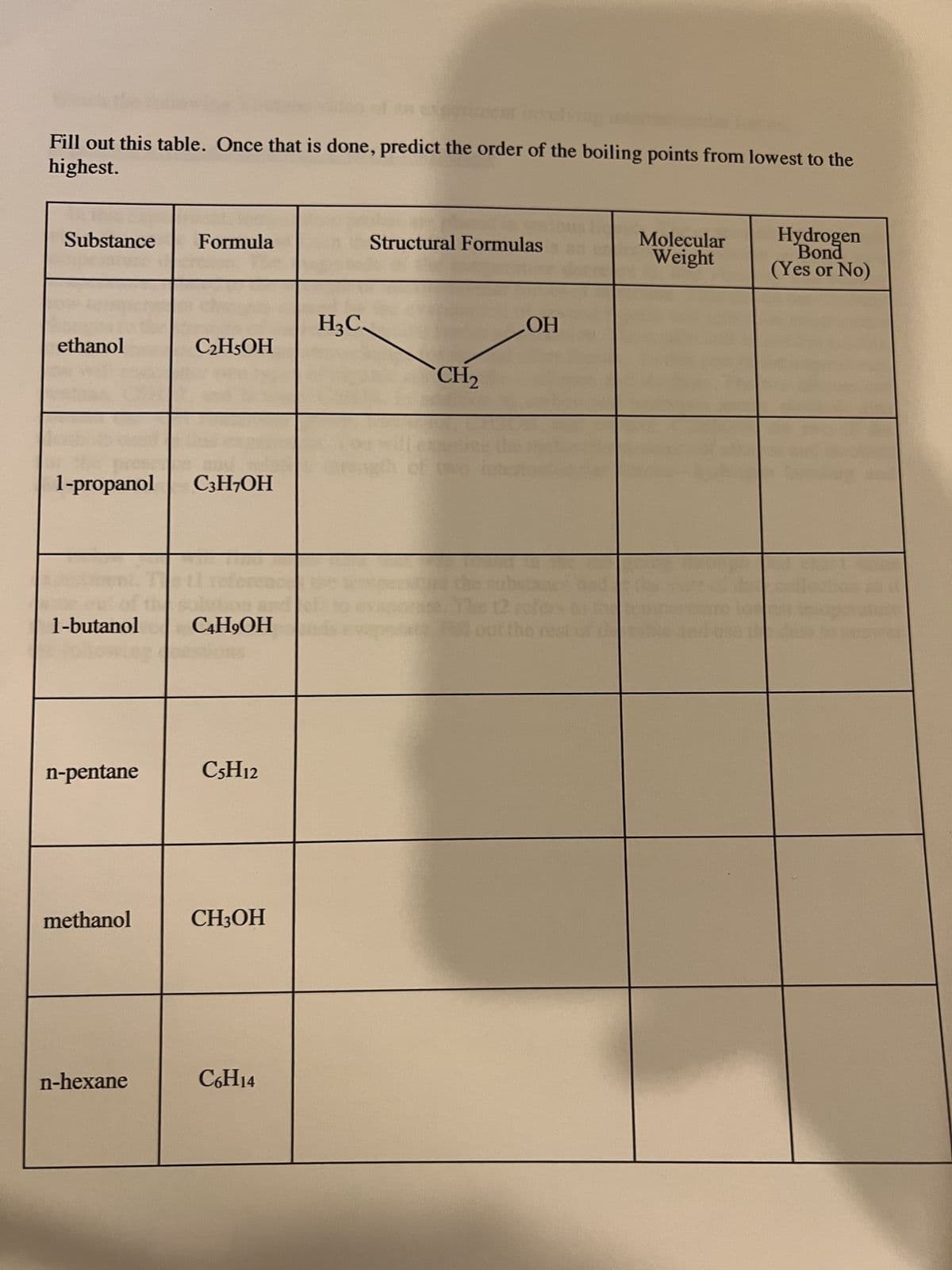
Transcribed Image Text:Fill out this table. Once that is done, predict the order of the boiling points from lowest to the
highest.
Substance
ethanol
1-butanol
1-propanol C3H7OH
n-pentane
methanol
Formula
n-hexane
C₂H5OH
C4H9OH
C5H12
CH3OH
C6H14
H₂C.
Structural Formulas
CH₂
OH
out the
Molecular
Weight
Hydrogen
Bond
(Yes or No)
dab
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning