Find the limiting factor in the Significant figures of your final calculated value for R. How many significant figures does it limit your final answer to? Using the data given
Find the limiting factor in the Significant figures of your final calculated value for R. How many significant figures does it limit your final answer to? Using the data given
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.8QAP
Related questions
Question
Find the limiting factor in the Significant figures of your final calculated value for R. How many significant figures does it limit your final answer to?
Using the data given
Transcribed Image Text:CSN Essay 1 o
Watch Euphoria 20... E Attack of the scabie...
Research and Citati..
1 / 2
100%
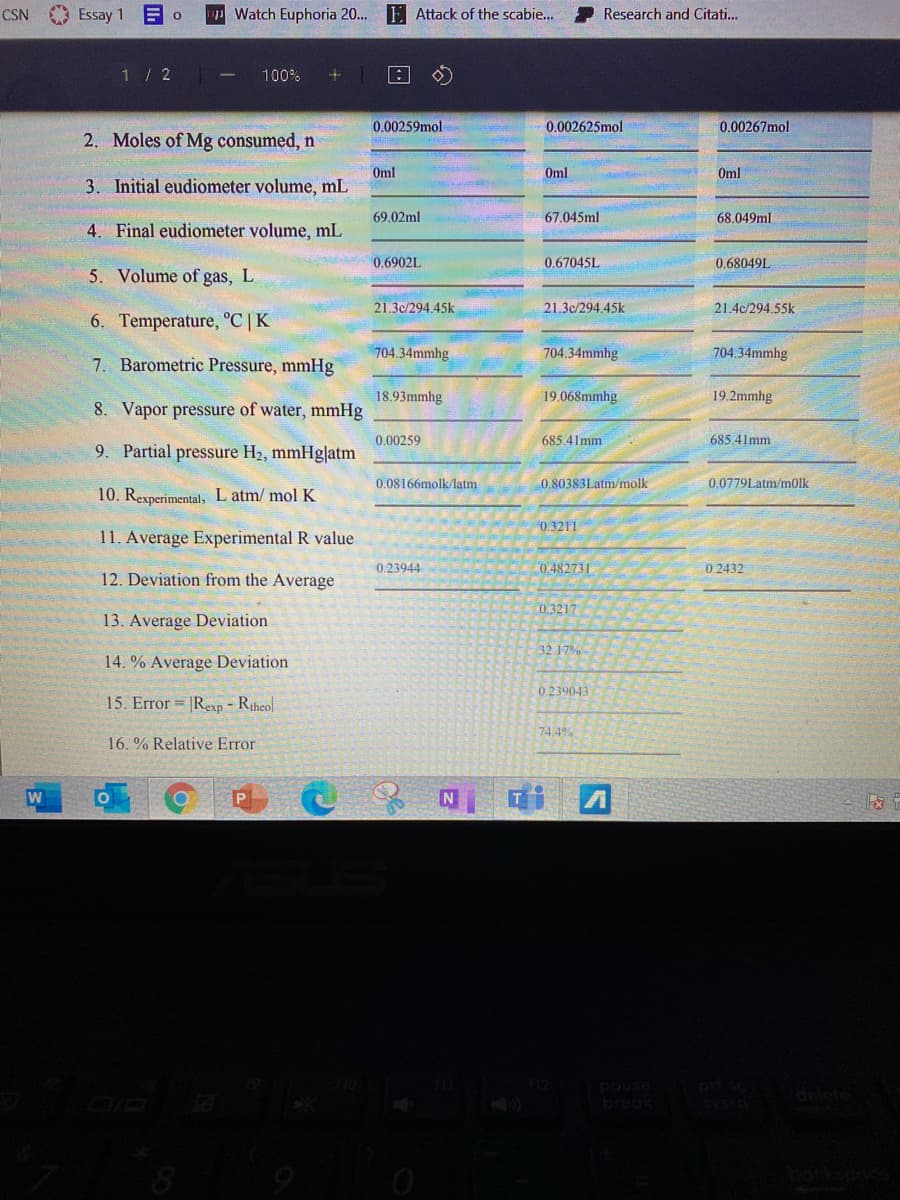
0.00259mol
0.002625mol
0.00267mol
2. Moles of Mg consumed, n
Oml
Oml
Oml
3. Initial eudiometer volume, mL
69.02ml
67.045ml
68.049ml
4. Final eudiometer volume, mL
0.6902L
0.67045L
0.68049L
5. Volume of gas, L
21.3c/294.45k
21.3c/294.45k
21.4c/294.55k
6. Temperature, °C | K
704.34mmhg
704.34mmhg
704.34mmhg
7. Barometric Pressure, mmHg
18.93mmhg
19.068mmhg
19.2mmhg
8. Vapor pressure of water, mmHg
0.00259
685.41mm
685.41mm
9. Partial pressure H2, mmHg|atm
0,08166molk/latm
0.80383Latm/molk
0.0779Latm/mOlk
10. Rexperimental, L atm/ mol K
0.3211
11. Average Experimental R value
0.23944
0.482731
0.2432
12. Deviation from the Average
0.3217
13. Average Deviation
32.17%
14. % Average Deviation
0.239043
15. Error = |Rexp - Riheol
74,4%
16. % Relative Error
prt s
pouse
breok
delete
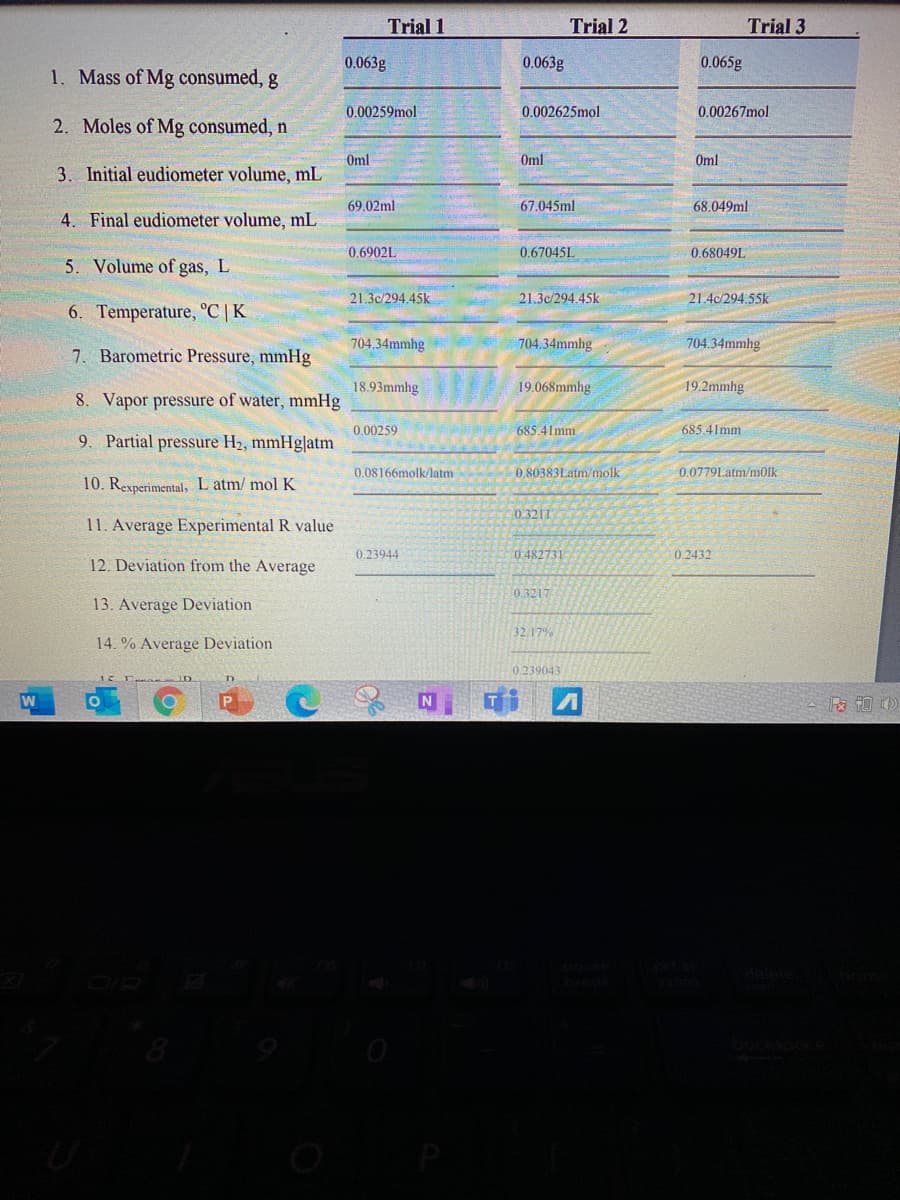
Transcribed Image Text:Trial 1
Trial 2
Trial 3
0.063g
0.063g
0.065g
1. Mass of Mg consumed, g
0.00259mol
0.002625mol
0.00267mol
2. Moles of Mg consumed, n
Oml
Oml
Oml
3. Initial eudiometer volume, mL
69.02ml
67.045ml
68.049ml
4. Final eudiometer volume, mL
0.6902L
0.67045L
0.68049L
5. Volume of gas, L
21.30/294.45k
21.3c/294.45k
21.4c/294.55k
6. Temperature, °C | K
704.34mmhg
704.34mmhg
704.34mmhg
7. Barometric Pressure, mmHg
18.93mmhg
19.068mmhg
19.2mmhg
8. Vapor pressure of water, mmHg
0.00259
685.41mm
685.41mm
9. Partial pressure H2, mmHg|atm
0.08166molk/latm
0.80383Latm/molk
0.0779Latm/m0lk
10. Rexperimental, L atm/ mol K
0.3211
11. Average Experimental R value
0.23944
0 482731
0.2432
12. Deviation from the Average
0.3217
13. Average Deviation
32 17%
14. % Average Deviation
0.239043
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
