Find the number of moles of water that can be formed if you have 114 mol of hydrogen gas and 62 mol of oxygen gas Express your answer with the appropriate units. View Available Hints) Value Units
Find the number of moles of water that can be formed if you have 114 mol of hydrogen gas and 62 mol of oxygen gas Express your answer with the appropriate units. View Available Hints) Value Units
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 49RGQ: Fluoridation of city water supplies has been practiced in the United States for several decades. It...
Related questions
Question
Please solve.
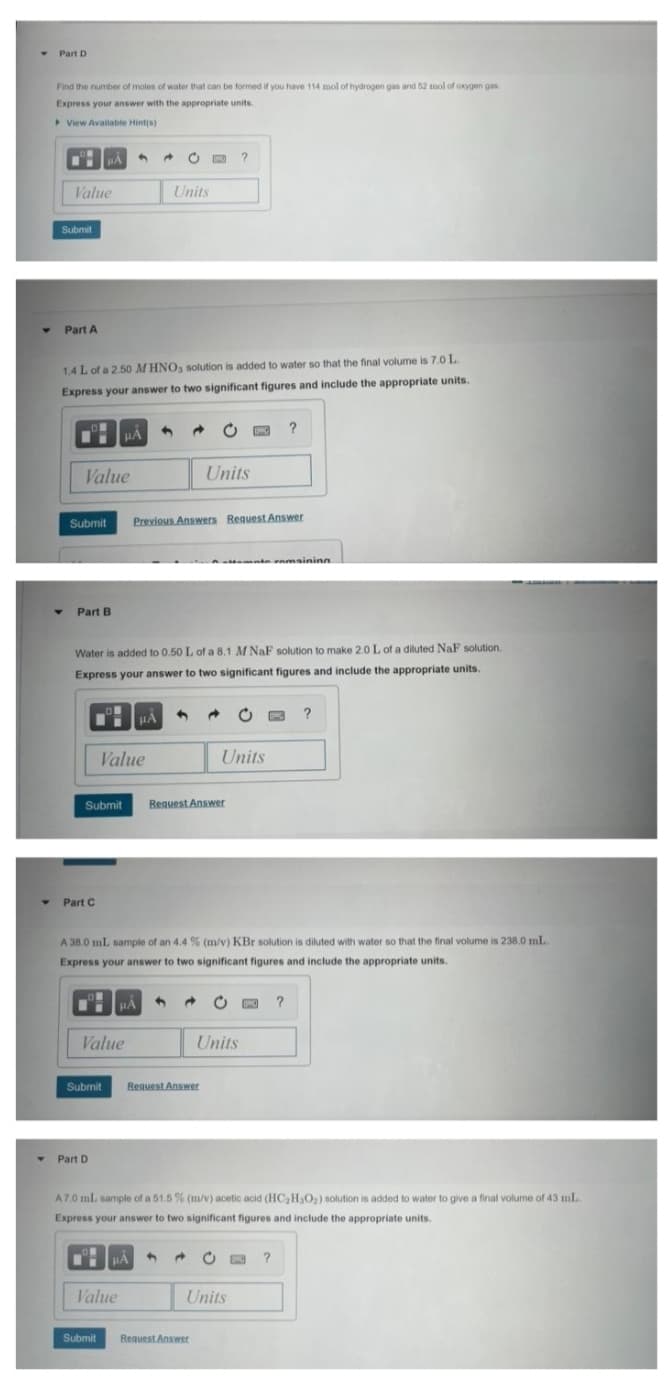
Transcribed Image Text:Part D
Find the number of moles of water that can be formed if you have 114 mol of hydrogen gas and 52 mol of oxygen gas
Express your answer with the appropriate units.
> View Available Hint(s)
Value
Units
Submit
Part A
1.4 L of a 2.50 M HNO, solution is added to water so that the final volume is 7.0 L.
Express your answer to two significant figures and include the appropriate units.
?
HA
Value
Units
Submit
Previous Answers Request Answer
nta amaininn
Part B
Water is added to 0.50 L of a 8.1 M NaF solution to make 2.0 L of a diluted NaF solution.
Express your answer to two significant figures and include the appropriate units.
HA
?
Value
Units
Submit
Reguest Answer
Part C
A 38.0 mL sample of an 4.4 % (m/v) KBr solution is diluted with water so that the final volume is 238.0 mL
Express your answer to two significant figures and include the appropriate units.
HA
?
Value
Units
Submit
Reguest Answer
Part D
A7.0 mL sample of a 51.5 % (m/v) acetic acid (HC2H3O2) solution is added to water to give a final volume of 43 mL
Express your answer to two significant figures and include the appropriate units.
HẢ
Value
Units
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning