Find the valence electrons of each molecule, the lewis dot structure, the shape and bond angle, VSPR with polar bonds, and molecular polarity. The answer should be the same as the example below
Find the valence electrons of each molecule, the lewis dot structure, the shape and bond angle, VSPR with polar bonds, and molecular polarity. The answer should be the same as the example below
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section3.4: Aqueous Solutions
Problem 1RC
Related questions
Question
Find the valence electrons of each molecule, the lewis dot structure, the shape and bond angle, VSPR with polar bonds, and molecular polarity. The answer should be the same as the example below
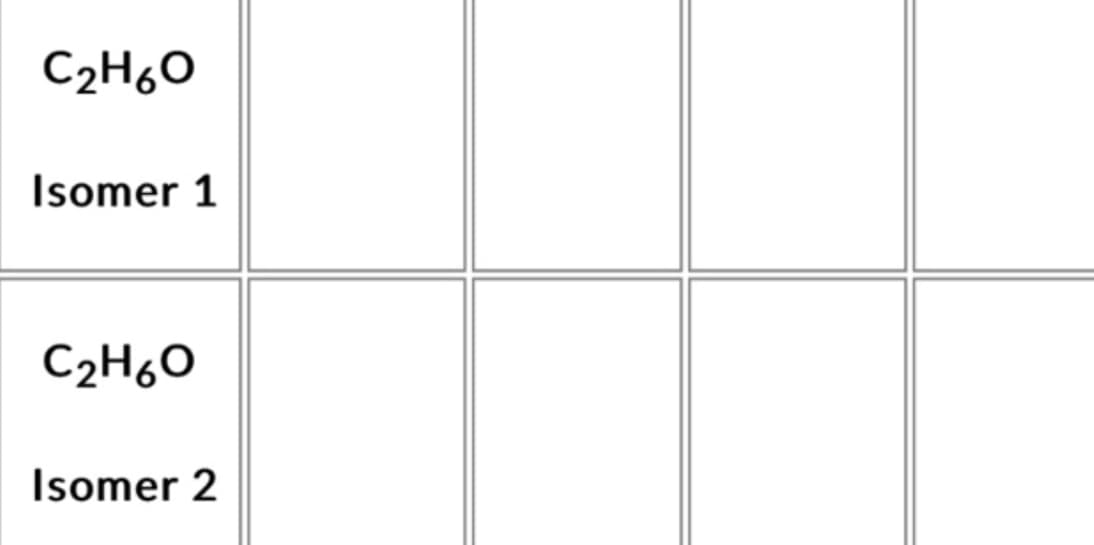
Transcribed Image Text:C₂H6O
Isomer 1
C₂H₂O
Isomer 2
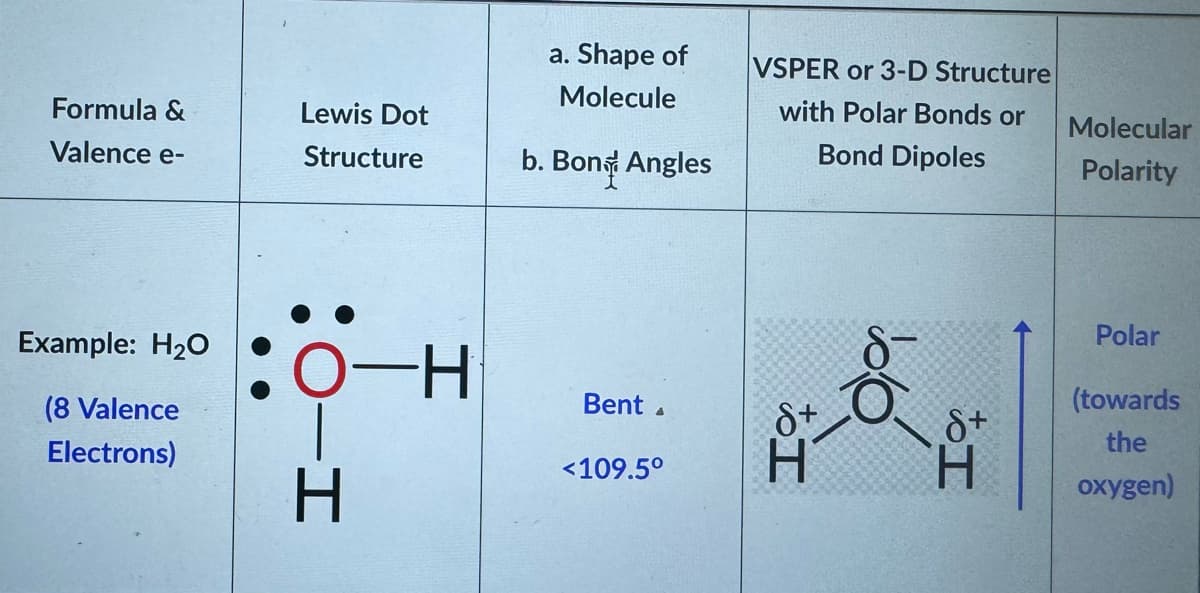
Transcribed Image Text:Formula &
Valence e-
Example: H₂O
(8 Valence
Electrons)
Lewis Dot
Structure
:O-H
O H
a. Shape of
Molecule
b. Bony Angles
Bent
<109.5°
VSPER or 3-D Structure
with Polar Bonds or
Bond Dipoles
8+
H
to I
Molecular
Polarity
Polar
(towards
the
oxygen)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 19 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning