find the Volume and temperature for a?
Related questions
Question
How do I find the Volume and temperature for a?
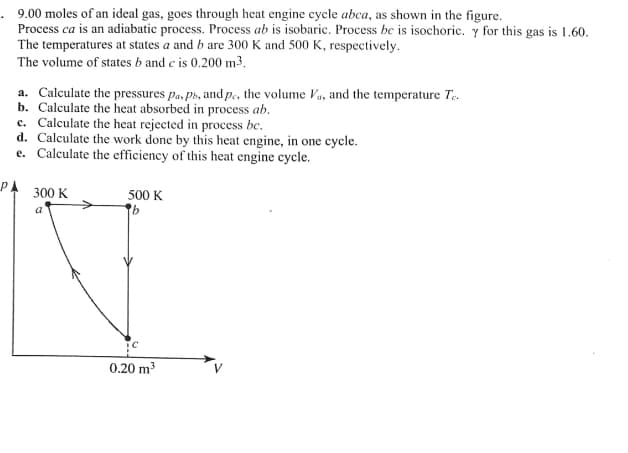
Transcribed Image Text:. 9.00 moles of an ideal gas, goes through heat engine cycle abca, as shown in the figure.
Process ca is an adiabatic process. Process ab is isobaric. Process be is isochoric. y for this gas is 1.60.
The temperatures at states a and b are 300 K and 500 K, respectively.
The volume of states b and c is 0.200 m3,
a. Calculate the pressures pa, ph, and p., the volume Va, and the temperature Te.
b. Calculate the heat absorbed in process ab.
c. Calculate the heat rejected in process bc.
d. Calculate the work done by this heat engine, in one cycle.
e. Calculate the efficiency of this heat engine cycle.
PA
300 K
500 K
a
0.20 m3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images
