Following a standardization reaction, the concentration of an aqueous solution of NaOH is determined to be 0.4510 M. a. What is the mass of NaOH in 50.0 mL of this solution? Show your work. b. What mass of KHP would be needed to completely react with 50.0 mL of this NAOH solution? Show your work.
Following a standardization reaction, the concentration of an aqueous solution of NaOH is determined to be 0.4510 M. a. What is the mass of NaOH in 50.0 mL of this solution? Show your work. b. What mass of KHP would be needed to completely react with 50.0 mL of this NAOH solution? Show your work.
Chapter9: Complexometric And Precipitation Titrations
Section: Chapter Questions
Problem 17P
Related questions
Question
I need help with questions 3a and 3b
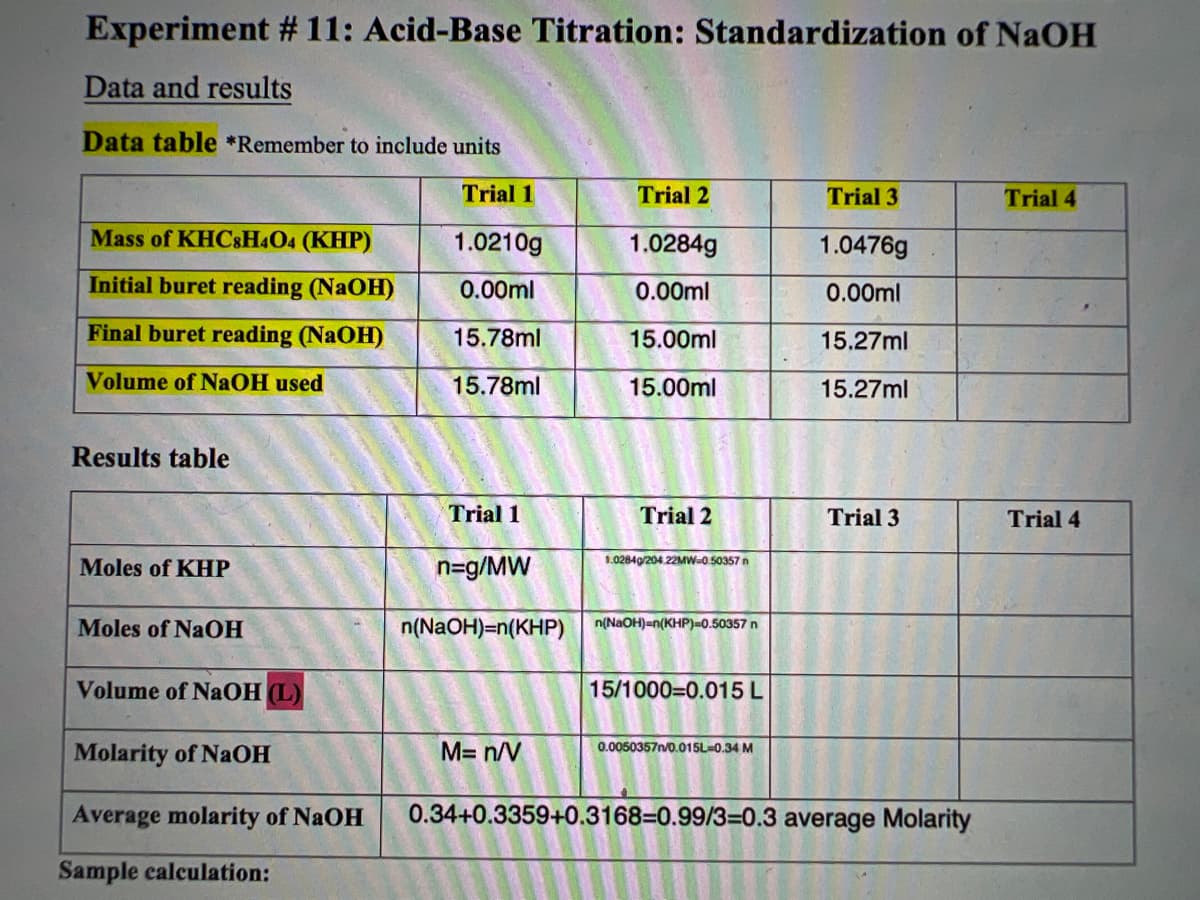
Transcribed Image Text:Experiment # 11: Acid-Base Titration: Standardization of NaOH
Data and results
Data table *Remember to include units
Trial 1
Trial 2
Trial 3
Trial 4
Mass of KHC&H4O4 (KHP)
1.0210g
1.0284g
1.0476g
Initial buret reading (NaOH)
0.00ml
0.00ml
0.00ml
Final buret reading (NAOH)
15.78ml
15.00ml
15.27ml
Volume of NAOH used
15.78ml
15.00ml
15.27ml
Results table
Trial 1
Trial 2
Trial 3
Trial 4
1.0284g/204.22MW-0.50357 n
Moles of KHP
n=g/MW
Moles of NaOH
n(NaOH)=n(KHP)
n(NaOH)=n(KHP)=0.50357 n
Volume of NaOH (L)
15/1000=0.015 L
Molarity of NaOH
M= n/V
0.0050357n/0.015L=0.34 M
Average molarity of NaOH
0.34+0.3359+0.3168=0.99/3=0.3 average Molarity
Sample calculation:

Transcribed Image Text:3. Following a standardization reaction, the concentration of an aqueous solution of NaOH is
determined to be 0.4510 M.
a. What is the mass of NaOH in 50.0 mL of this solution? Show your work.
b. What mass of KHP would be needed to completely react with 50.0 mL of this NAOH
solution? Show your work.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



