For a trip of 750 miles (in the United States), how many gallons of fuel does (a) the mistaken tourist believe she needs and (b) the car actually require?
For a trip of 750 miles (in the United States), how many gallons of fuel does (a) the mistaken tourist believe she needs and (b) the car actually require?
College Physics
11th Edition
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter1: Units, Trigonometry. And Vectors
Section: Chapter Questions
Problem 74AP: Assume it takes 7.00 minutes to fill a 30.0-gal gasoline tank. (a) Calculate the rate at which the...
Related questions
Question
Hi I need help with this question how can I solve I need to learn step by step please
1- give quantities
2 sketch the situation in the problem
3 unknown quantities
4 principles key ideas
5 Equation
6 analytic solution
7 númeric solution
8final answer
Unit check
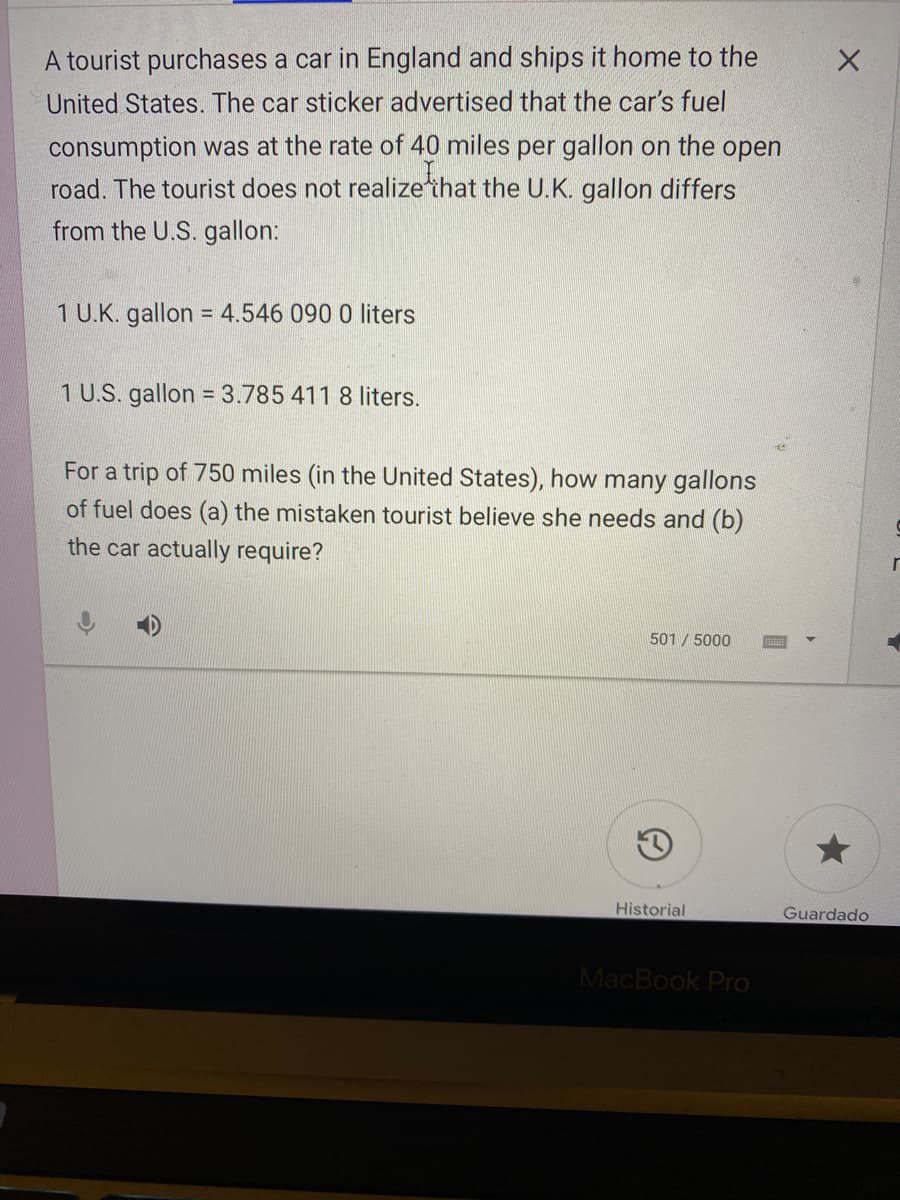
Transcribed Image Text:A tourist purchases a car in England and ships it home to the
United States. The car sticker advertised that the car's fuel
consumption was at the rate of 40 miles per gallon on the open
road. The tourist does not realize'that the U.K. gallon differs
from the U.S. gallon:
1 U.K. gallon = 4.546 090 0 liters
1 U.S. gallon = 3.785 411 8 liters.
For a trip of 750 miles (in the United States), how many gallons
of fuel does (a) the mistaken tourist believe she needs and (b)
the car actually require?
501 / 5000
Historial
Guardado
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning