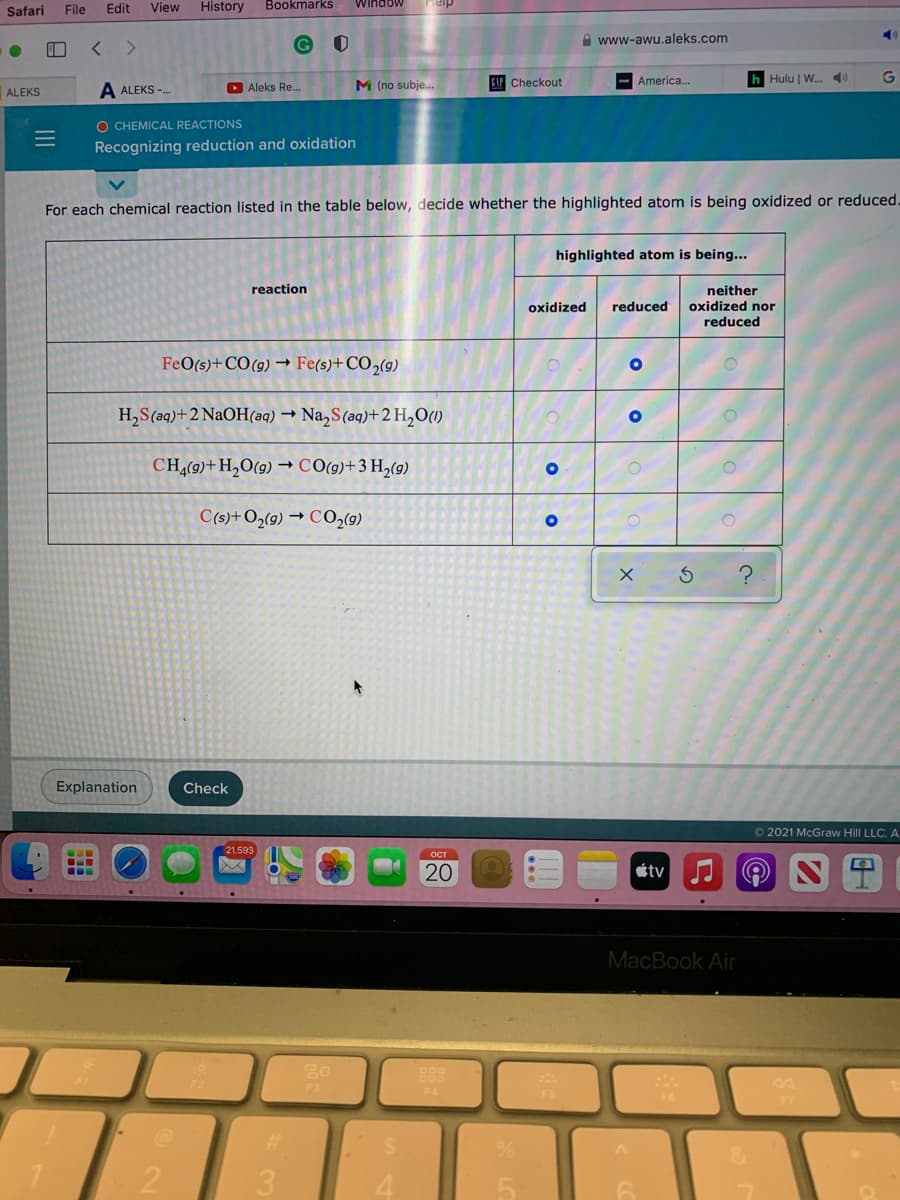
For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced. highlighted atom is being... reaction neither oxidized nor reduced oxidized reduced FeO(s)+CO(9) → Fe(s)+CO2(9) H,S(aq)+ 2 NaOH(aq) → Na, S(aq)+ 2 H,O(1) CH(9)+H,O(9) CO(g)+3 H,(9) C(s)+O2(9) → CO,(9)
For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced. highlighted atom is being... reaction neither oxidized nor reduced oxidized reduced FeO(s)+CO(9) → Fe(s)+CO2(9) H,S(aq)+ 2 NaOH(aq) → Na, S(aq)+ 2 H,O(1) CH(9)+H,O(9) CO(g)+3 H,(9) C(s)+O2(9) → CO,(9)
Chapter19: Applications Of Standard Electrode Potentials
Section: Chapter Questions
Problem 19.7QAP
Related questions
Question
Recognizing reduction and oxidation
Transcribed Image Text:Safari
File
Edit
View
History
Bookmarks
Window
G
A www-awu.aleks.com
D Aleks Re.
M (no subje...
CAP Checkout
America..
h Hulu | W. 4)
ALEKS
A ALEKS -.
O CHEMICAL REACTIONS
Recognizing reduction and oxidation
For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced.
highlighted atom is being...
reaction
neither
oxidized nor
reduced
oxidized
reduced
FeO(s)+CO(g) → Fe(s)+CO2(g)
H,S(aq)+2 NaOH(aq) → Na,S(aq)+2 H,O(1)
CH,(9)+H,O(9) → CO(9)+3 H,(9)
C(s)+O2(9) → CO,(9)
Explanation
Check
O 2021 McGraw Hill LLC. A
21,593
ост
étv
MacBook Air
F4
20
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax