For each equilibrium below, describe the situation at equilibrium. Is it mostly products, mostly reactants or are both reactants and products present in reasonable amounts (not necessarily equal) at equilibrium? For full credit, give a brief explanation.b. PCl5(g) ⇄ PCl3(g) + Cl2(g) Kc = 0.030
For each equilibrium below, describe the situation at equilibrium. Is it mostly products, mostly
reactants or are both reactants and products present in reasonable amounts (not necessarily equal)
at equilibrium? For full credit, give a brief explanation.
b. PCl5(g) ⇄ PCl3(g) + Cl2(g) Kc = 0.030
The nature of equilibrium reactions is governed with their equilibrium constant values that is simply the proportion of products-reactants' amount at final stage. This ratio is also helpful in finding any unknown species concentration after reaction's completion.
The given reaction is as follows,
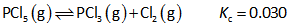
The equilibrium constant expression for any reaction is shown below.
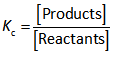
Since, the value of Kc is less than 1 which is possible only when reactants’ amount at equilibrium exceeds the products’ amount.
Therefore,
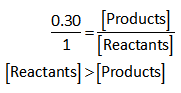
Hence, at equilibrium, the concentration of reactants is larger in comparison to the concentration of products.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images









