Q: ising the temperature b. Adding a catalyst c. Increasing the concentration of the reactants d.…
A: Chemical kinetics deals with the changes in the rates of the reaction with changes in the other…
Q: The act of striking a match illustrates the role of activation energyin a chemical reaction.…
A: The act of striking a match illustrates the role of activation energyin a chemical reaction.
Q: Catalysts are correctly characterized by each of the following statements except one. The exception…
A:
Q: 7. Consider the following graphs of In k versus 1/T for reactions A, B, C, and D. D In k In k 1 1 I…
A: According to Arrhenius equation activation energy is determined by the negative slope of the graph.
Q: For the following reaction profile, indicate E Reaction coordinate a) the positions of reactants and…
A: a) The initial component of the curve shows the reactant and final shows products hence the…
Q: Distinguish between reaction order and molecularity.
A: To differentiate between reaction order and molecularity.
Q: 11. If reaction A has an activation energy of 250 kJ and reaction B has an activation energy of 100…
A:
Q: tion energy for the reverse reaction is 25.4 kJ. What is the
A: Firstly we should know about reaction nature. This reaction is Endothermic because of breaking of…
Q: Please explain the three different ways of catalysis Acid/base Covalent Metal iron
A: The three types of catalysis are discussed below:
Q: B Reaction Coordinate /hat does arrow 'C' represent? the heat of reaction for the forward reaction…
A: Activation energy is defined as the minimum amount of energy required by the reacting molecules to…
Q: grade 12 chemistry: How would you explain the concept of activation energy to someone who has only…
A:
Q: Which of the following energy diagrams shows the reaction with the largest energy of activation?…
A:
Q: Q1) Which of the following statements regarding collision and transition state theories b)…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: A reaction has an activation energy of 15 kJ mol. The temperature of the reaction must be increased…
A:
Q: In the Haber process, nitrogen and hydrogen react together to produce ammonia, NH3 according to the…
A: Given reaction is : N2 (g) + 3H2 (g) <---------> 2NH3 (g) Enthalpy of the reaction = ΔH =…
Q: What is activation energy for the reaction B → A in the following diagram? R Cd D C OA
A: The minimum energy that is required to form the product from the starting material is termed as…
Q: Which of the following would change the value of the activation energy for a heterogeneous reaction?…
A: The catalyst is a chemical species that increases the rate of a chemical reaction and it does not…
Q: In the following chemical reaction at a certain temperature, if you know that the potential energy…
A:
Q: Which of the following conclusions concerning the concentration-time plot provided below is/are…
A: Chemical kinetics is branch of chemistry in which we deal with rate of reaction.
Q: Which of the following statements are true? I. Activation energy of a reaction is smaller at higher…
A: Activation energy: The excess energy that the reactant molecules having energy less than the…
Q: Which of the following can lower the activation energy for a reaction? A. Increasing the…
A: To determine which of the given option lower the activation energy of a reaction.
Q: Given that a reaction is Exothermic in the foward direction and has a forward activation energy of…
A: Exothermic reaction is the reaction where heat is released in the reaction. Activation energy is the…
Q: The lower reaction path, including points B, C and D, could represent which of the following things:…
A:
Q: what is the effect on k as the activation energy for a reaction increases
A: The Arrhenius equation is given as :
Q: To complete the following statement: To speed up the reaction, a catalyst... A) increases the…
A: The main role of catalyst is to bring a short way for any certain reaction to take place. Therefore,…
Q: Given that a reaction is Exothermic in the foward direction and has a forward activation energy of…
A:
Q: Which of the following changes will affect the activation energy of a reaction? 1. Increasing or…
A: Activation energy: When the reactant molecules convert into the products, they required the minimum…
Q: (b) Explain what will happen to the activation energy and pre-exponential factor if the following…
A: In a chemical reaction, the conversion of reactants to products requires several criteria to be met.…
Q: 2. Describe the following quantities: activation energy (Ea), barrier height (Eb), threshold energy…
A: Answer is given below
Q: State the role of activated complex in a reaction and state its relation withactivation energy.
A: The collision of two molecules could result in the chemical reaction which is done by an activated…
Q: Examine the 2-step mechanism below and identify the substance that appears to be a catalyst and an…
A: The given reaction has two-step mechanism.
Q: Define the term initial rate of reaction
A: Term = Initial rate Definition = ?
Q: Reaction A has a high activation energy, whereas reacton B has a low activation energy. Which of the…
A: Given:- Reaction A has high activation energy, whereas reaction B has lower activation energy. We…
Q: Draw an energy pathway diagram for the following reaction. Label the axes, reactants, products,…
A: Here in the given reaction Carbon and oxygen are the reactants and carbon dioxide is the product.
Q: What is the difference between an activated complex and a transition state?
A: The pathway of the progress of an organic reaction will be explained by the reaction coordinate…
Q: 7. When a catalyst is added to a reaction the activation Energy is (increased or decreased), (more…
A: The above statement is related to a catalyst. A catalyst is a substance which increase the rate of…
Q: (b) Explain what will happen to the activation energy and pre-exponential factor if the following…
A: The Answer to the following question is -
Q: c. Draw a reaction diagram for the reaction between C27H29OSS and NH4CI. Label the enthalpy,…
A: Given: Reaction of thymol blue with ammonium chloride To find: Energy profile diagram Solution:…
Q: For the following reaction profile, indicate a. the positions of reactants and products. b. the…
A: The reaction coordinate diagram shows the energy of the reactants, products, and the transition…
Q: Which of the following are ways that catalysts can speed up reactions rates? You must check all…
A: A catalyst is a chemical substance that can be added to a reaction, without being absorbed in the…
Q: What is the rate constant for the reaction?
A: Rate constant is the proportionality constant in the rate law. Rate law is the mathematical…
Q: 2c) Briefly describe what effect, if any, a catalyst has on the activation energy and the net free…
A: A catalyst: alters the rate of reaction without being consumed in the reaction. Catalyst have effect…
Q: (T/I=4)In terms of reaction kinetics, explain why each of the following increases reaction rate: (a)…
A: We have to explain the reason why the reaction rate increase with the temperature. The rate of any…
Q: Briefly comment on the effect of a catalyst on each of the following: (a) activation energy, (b)…
A: Catalyst is one that can't be consumed in the reaction at the end reaction. However, it can change…
Q: explain the difference between the net energy change of a reaction and the activation energy of the…
A: Activation Energy (Ea)= Activation energy is defined as the energy difference between the potential…
Q: Write two differences between ‘order of reaction’ and ‘molecularity of reaction’.
A: Reaction order: It refers to the power dependence of the rate on the concentration of each reactant.…
Q: Which of the following statements is false? Lowering the activation energy increases the rate of a…
A: We know that: Activation energy is the minimum amount of energy that is required to activate atoms…
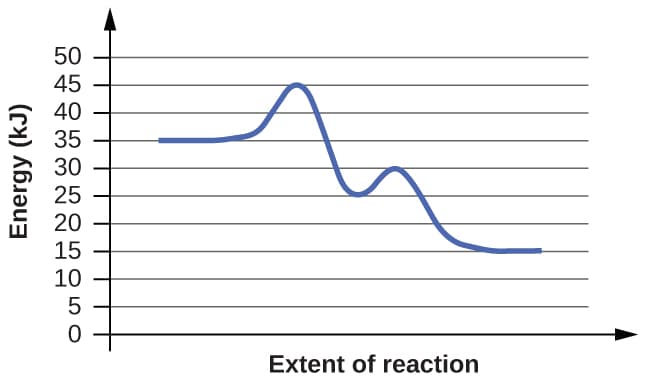
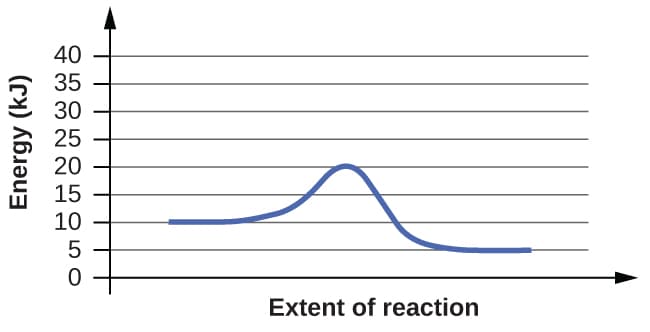
For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
(a)
(b)
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

- The same student wanted to determine the heat of dissolution for potassium chlorate (KClO3, MW: 122.55 g/mol) in water. He measured 10 grams of KClO3 and dissolved it in a calorimeter containing 250 g of water. The temperature drop was 3.5 K. What is q of rxn?Use ∆Hf0 of CO2 and H2O and combustion enthalpy of sucrose (6.155 x 103 kJmol-1 ) to calculate ∆Hf0 of sucroseThe same student wanted to determine the heat of dissolution for potassium chlorate (KClO3, MW: 122.55 g/mol) in water. He measured 10 grams of KClO3 and dissolved it in a calorimeter containing 250 g of water. The temperature drop was 3.5 K. What is qH2O? What is qrxn? What is ΔHrxn?
- The same student wanted to determine the heat of dissolution for potassium chlorate (KClO3, MW: 122.55 g/mol) in water. He measured 10 grams of KClO3 and dissolved it in a calorimeter containing 250 g of water. The temperature drop was 3.5 K. What is qH2O in Joules? What is qdissolution in Joules? What is the ΔHdissolution in kilojoules per mole? What is the ΔHdissolution in kilojoules per mole?Calculate the standard enthalpy change for the fermentation process, in which glucose (C6H12O6) is converted into ethanol (C2H5OH) and carbon dioxide (CO2). Substance: Enthalpy of Formation: CO2(g) -393.5 kJ/mol CO2(aq) -412.9 kJ/mol C2H5OH(l) -276.98 kJ/mol C6H12O6(s) -1,274.5 kJ/mol H2O(g) -241.8 kJ/mol H2O(l) -285.8 kJ/mol O2(g) 0 kJ/molThe same student wanted to determine the heat of dissolution for potassium chlorate (KClO3, MW: 122.55 g/mol) in water. He measured 10 grams of KClO3 and dissolved it in a calorimeter containing 250 g of water. The temperature drop was 3.5 K. What is qH2O? What is qrxn? What is ΔHrxn? Is the reaction endothermic or exothermic?
- Calculate Ksp for the salt NaCl at 25°C. Substance ΔGf°(in kJ/mol) Na+(aq) –262.0 Cl–(aq) –131.0 NaCl(s) -383.6The heat of combustion of carbon, hydrogen and sucrose are 394.82, -289.28 and -5665.8kj/mol resp. What is the enthalpy of formation of sucrose?An engineering student wants to study the heat transfer between a 50 g of a heated block of iron (cFe = 0.449 J/g ⁰C) at 500 ⁰C and 100 g of water (cH2O = 4.184 J/g ⁰C) at 10 ⁰C using a calorimeter. The same student wanted to determine the heat of dissolution for potassium chlorate (KClO3, MW: 122.55 g/mol) in water. He measured 10 grams of KClO3 and dissolved it in a calorimeter containing 250 g of water. The temperature drop was 3.5 K.
- The same student wanted to determine the heat of dissolution for potassium chlorate (KClO3, MW: 122.55 g/mol) in water. He measured 10 grams of KClO3 and dissolved it in a calorimeter containing 250 g of water. The temperature drop was 3.5 K. What is qH2O? What is q of rxn? What is ΔHrxn? Is the reaction endothermic or exothermic?What is the value of ΔG°rxn for the reaction: C --> 2A+BGiven: 2A+B --> C and ΔG°rxn = 260.5 kJ/molThe reaction of liquid methyl chloride (CH3Cl) with water produces methanol and hydrogen chloride gas at room temperature, despite the fact that ΔHo rxn = 28.3 kJ/mol. Using thermodynamic arguments, propose an explanation as to why methanol forms. CH3Cl (l) + H2O (l) → CH3OH (l) + HCl (g)

