For each of the following, write an oxidation half-reaction and normalize the reaction on an electron-equivalent basis. Add H,O as appropriate to either side of the equations in balancing the reactions: (a) CH CH CH CHNH COO oxidation to CO NH, and HCO; 5.18 2 2 (b) Cl- to CIO-
For each of the following, write an oxidation half-reaction and normalize the reaction on an electron-equivalent basis. Add H,O as appropriate to either side of the equations in balancing the reactions: (a) CH CH CH CHNH COO oxidation to CO NH, and HCO; 5.18 2 2 (b) Cl- to CIO-
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 10E: Why is it not possible for hydroxide ion (OH-) to appear in either of the half-reactions or the...
Related questions
Question
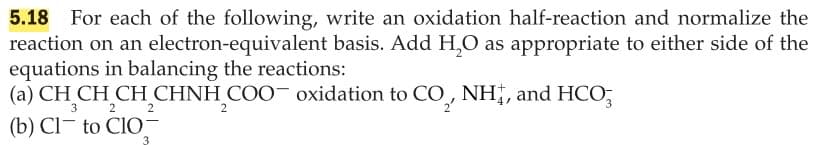
Transcribed Image Text:For each of the following, write an oxidation half-reaction and normalize the
reaction on an electron-equivalent basis. Add H,O as appropriate to either side of the
equations in balancing the reactions:
(a) CH CH CH CHNH COO oxidation to CO NH,, and HCO;
5.18
3
2
2
2
(b) Cl- to ClO-
Expert Solution
Step 1
Redox reaction is the reaction in which their is oxidation and reduction take place simultaneously means when one species is reduced and other get oxidised.
The gain of electron is known as reduction whereas loss of electron is known as oxidation.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning