For each of the substrates below, identify whether: (A) the rate of substitution doesn't depend on nucleophile concentration and (B) the product distribution from substitution gives a 50/50 mix of enantiomers. If you answered "yes" for the first susbtrate, draw the intermediate that forms during a nucleophilic substitution reaction in the space below the table.
For each of the substrates below, identify whether: (A) the rate of substitution doesn't depend on nucleophile concentration and (B) the product distribution from substitution gives a 50/50 mix of enantiomers. If you answered "yes" for the first susbtrate, draw the intermediate that forms during a nucleophilic substitution reaction in the space below the table.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 26Q: How can one construct a galvanic cell from two substances, each having a negative standard reduction...
Related questions
Question
For each of the substrates below, identify whether:
(A) the rate of substitution doesn't depend on nucleophile concentration and (B) the product distribution from substitution gives a 50/50 mix of enantiomers. If you answered "yes" for the first susbtrate, draw the intermediate that forms during a nucleophilic substitution reaction in the space below the table.
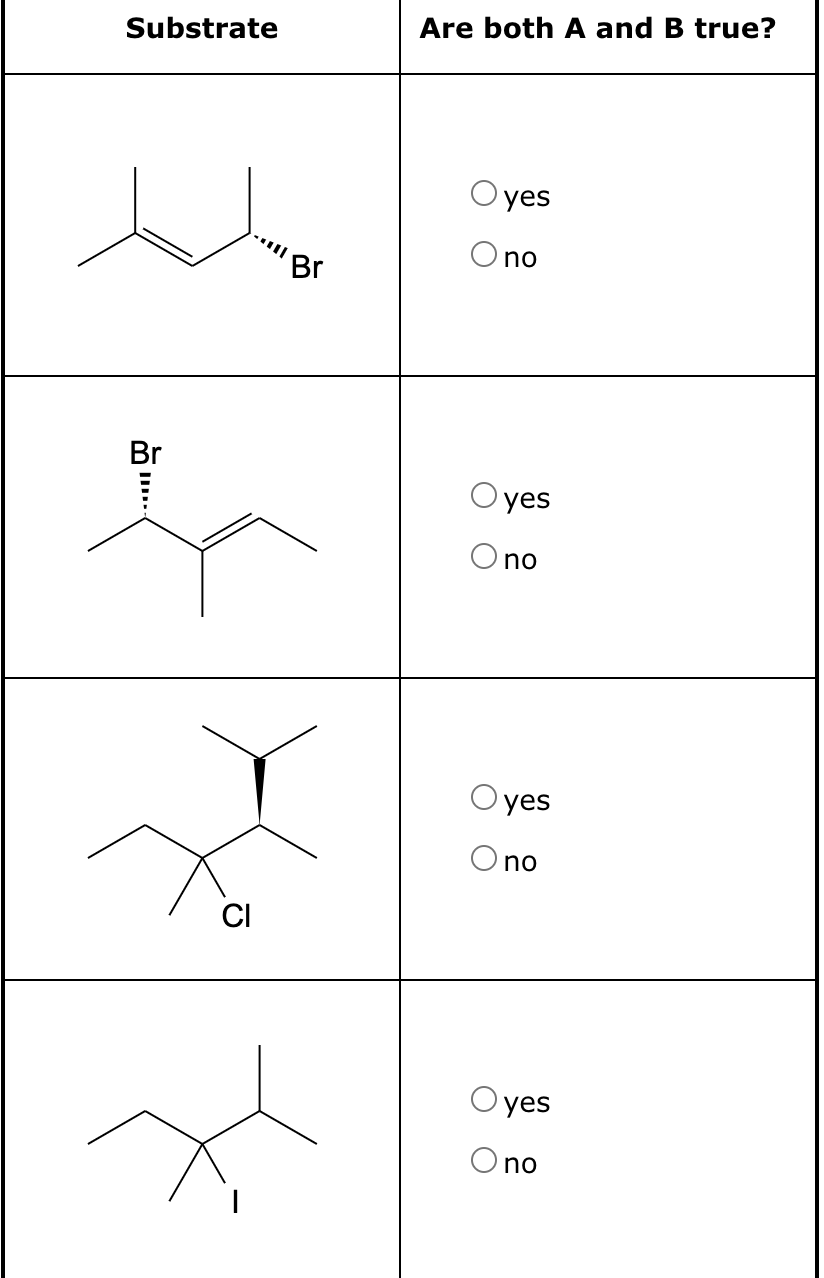
Transcribed Image Text:Substrate
Br
CI
Br
Are both A and B true?
O yes
O no
yes
O no
O yes
O no
Oyes
O no
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
