For many purposes we can treat ammonia (NH3 as an ideal gas at temperatures above its boiling point of-33. °C. Suppose the temperature of a sample of ammonia gas is raised from11.0 °C to 17.0 °C, and at the same time the pressure is changed. If the initial pressure was 4.9 atm and the volume increased by 60.0%, what is the final pressure? Round your answer to the correct number of significant digits. atm |x10 ? Submit Assignment Continue Terms of Use Prvacy 2019 McGraw-Hill Education. All Rights Reserved MacBook Pro DII FI2 OO0 O00 F10 F 9 F8 F7 F6 F5 F4 esc F3 F2 F1 % X
For many purposes we can treat ammonia (NH3 as an ideal gas at temperatures above its boiling point of-33. °C. Suppose the temperature of a sample of ammonia gas is raised from11.0 °C to 17.0 °C, and at the same time the pressure is changed. If the initial pressure was 4.9 atm and the volume increased by 60.0%, what is the final pressure? Round your answer to the correct number of significant digits. atm |x10 ? Submit Assignment Continue Terms of Use Prvacy 2019 McGraw-Hill Education. All Rights Reserved MacBook Pro DII FI2 OO0 O00 F10 F 9 F8 F7 F6 F5 F4 esc F3 F2 F1 % X
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 46QRT
Related questions
Question
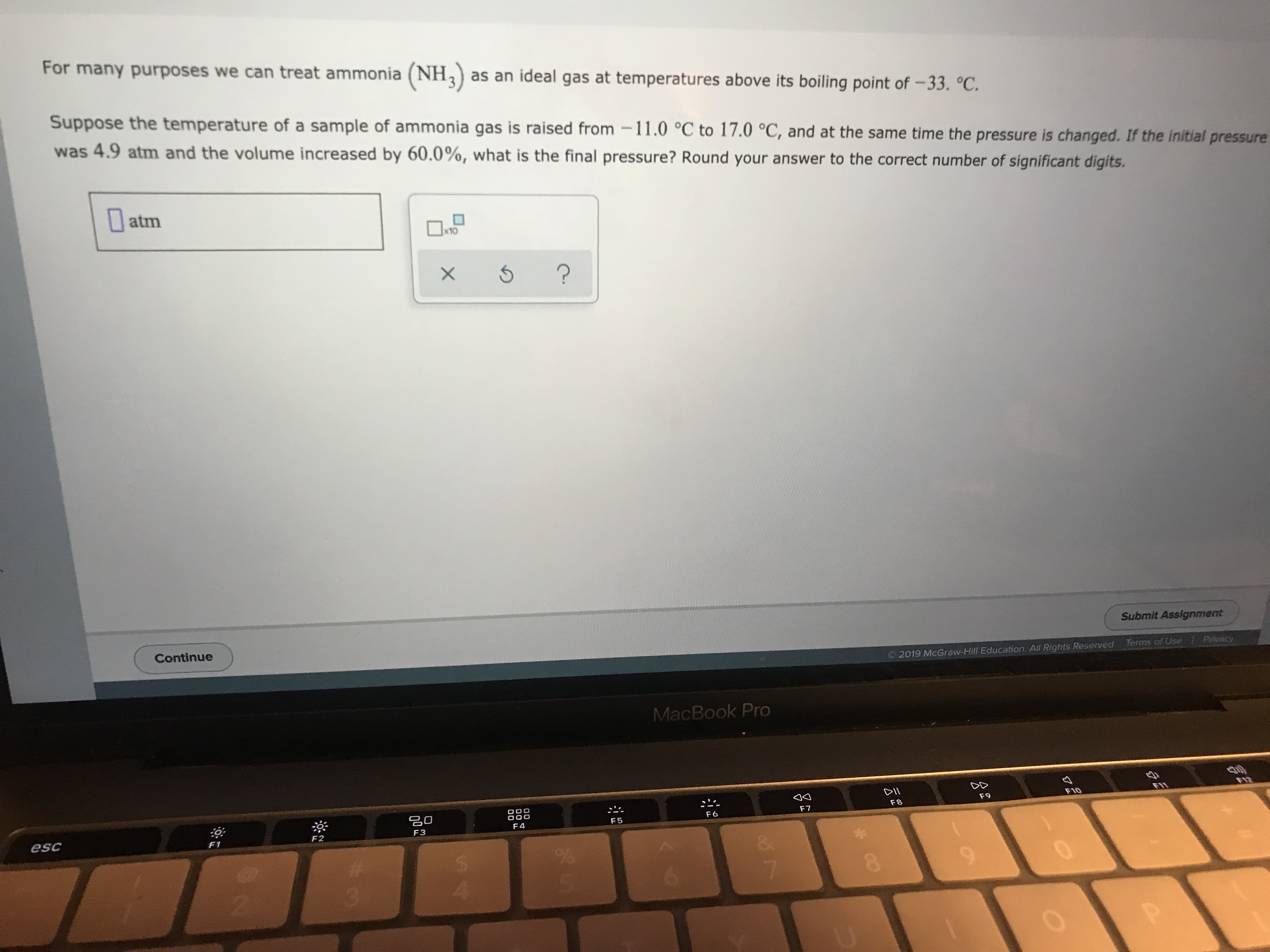
Transcribed Image Text:For many purposes we can treat ammonia (NH3 as an ideal gas at temperatures above its boiling point of-33. °C.
Suppose the temperature of a sample of ammonia gas is raised from11.0 °C to 17.0 °C, and at the same time the pressure is changed. If the initial pressure
was 4.9 atm and the volume increased by 60.0%, what is the final pressure? Round your answer to the correct number of significant digits.
atm
|x10
?
Submit Assignment
Continue
Terms of Use
Prvacy
2019 McGraw-Hill Education. All Rights Reserved
MacBook Pro
DII
FI2
OO0
O00
F10
F 9
F8
F7
F6
F5
F4
esc
F3
F2
F1
%
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning