For many purposes we can treat ammonia (NH3) as an ideal gas at temperatures above its boiling point of -33. °C. Suppose the pressure on a 830. mL sample of ammonia gas at 11.0°C is cut in half. Is it possible to change the temperature of the ammonia at the same time such that the volume of the gas doesn't change? If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C. O yes O no °C x10 X
For many purposes we can treat ammonia (NH3) as an ideal gas at temperatures above its boiling point of -33. °C. Suppose the pressure on a 830. mL sample of ammonia gas at 11.0°C is cut in half. Is it possible to change the temperature of the ammonia at the same time such that the volume of the gas doesn't change? If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C. O yes O no °C x10 X
Chapter5: Gases
Section: Chapter Questions
Problem 143CWP: A certain flexible weather balloon contains helium gas at a volume of 855 L. Initially, the balloon...
Related questions
Question
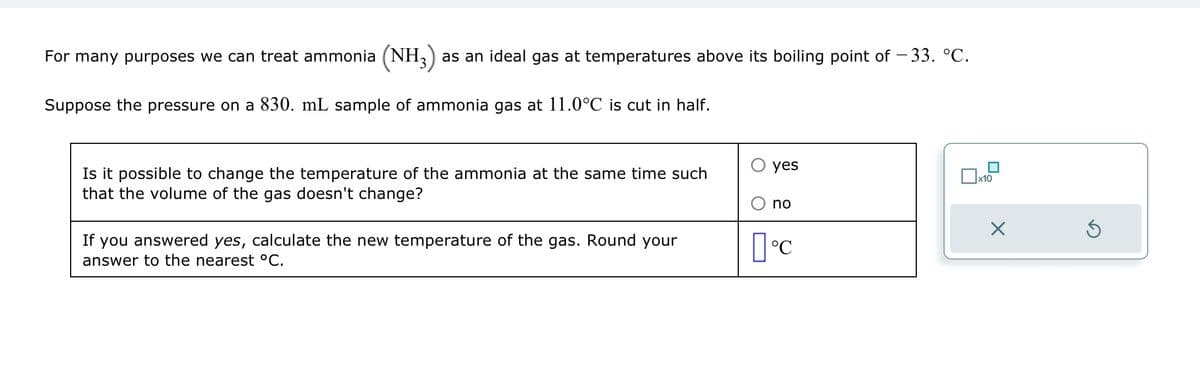
Transcribed Image Text:For many purposes we can treat ammonia (NH3) as an ideal gas at temperatures above its boiling point of – 33. °C.
Suppose the pressure on a 830. mL sample of ammonia gas at 11.0°C is cut in half.
Is it possible to change the temperature of the ammonia at the same time such
that the volume of the gas doesn't change?
If you answered yes, calculate the new temperature of the gas. Round your
answer to the nearest °C.
yes
no
°C
x10
X
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning