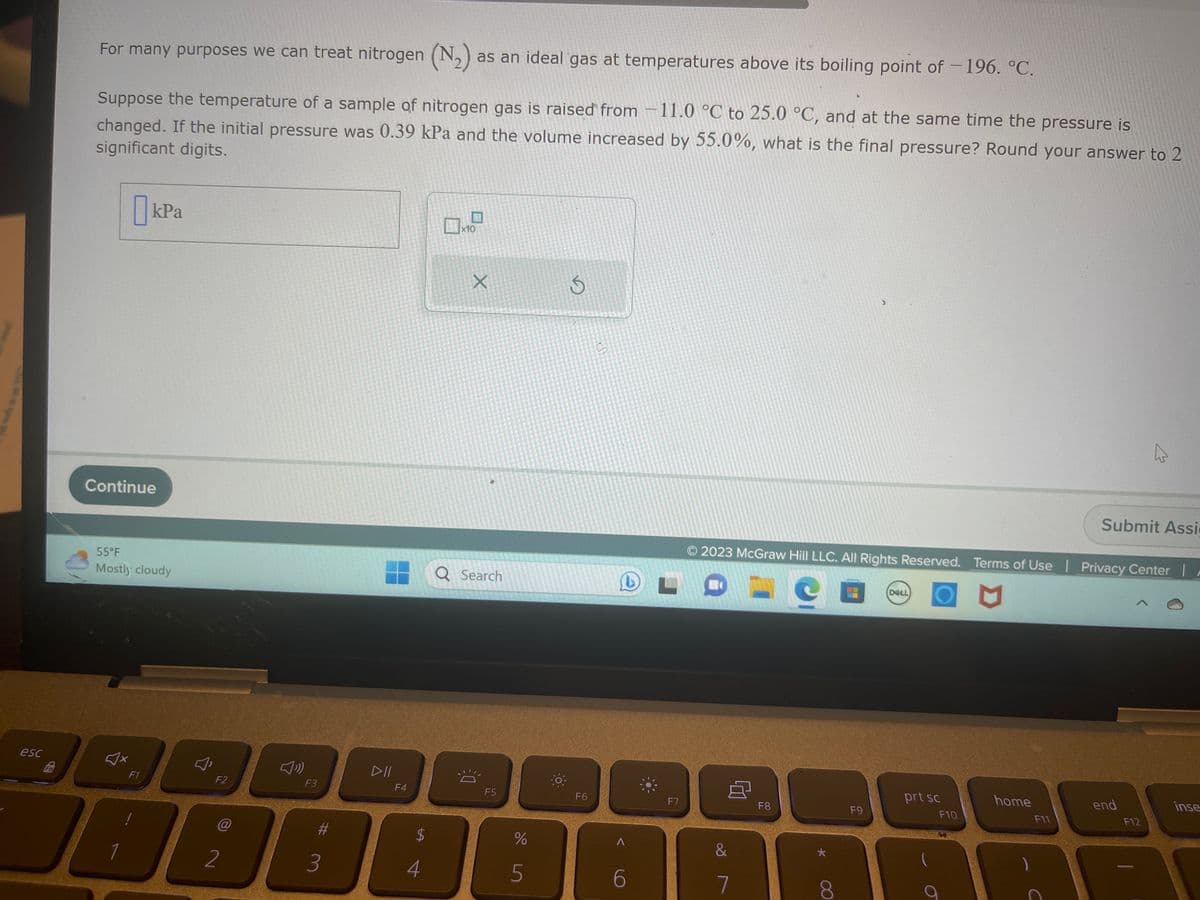
For many purposes we can treat nitrogen (N₂) as an ideal gas at temperatures above its boiling point of -196. °C. Suppose the temperature of a sample of nitrogen gas is raised from 11.0 °C to 25.0 °C, and at the same time the pressure is changed. If the initial pressure was 0.39 kPa and the volume increased by 55.0%, what is the final pressure? Round your answer to 2 significant digits. kPa X
For many purposes we can treat nitrogen (N₂) as an ideal gas at temperatures above its boiling point of -196. °C. Suppose the temperature of a sample of nitrogen gas is raised from 11.0 °C to 25.0 °C, and at the same time the pressure is changed. If the initial pressure was 0.39 kPa and the volume increased by 55.0%, what is the final pressure? Round your answer to 2 significant digits. kPa X
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter7: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 7.32EP
Related questions
Question
Transcribed Image Text:esc
Fn
For many purposes we can treat nitrogen (N₂) as an ideal gas at temperatures above its boiling point of -196. °C.
Suppose the temperature of a sample of nitrogen gas is raised from 11.0 °C to 25.0 °C, and at the same time the pressure is
changed. If the initial pressure was 0.39 kPa and the volume increased by 55.0%, what is the final pressure? Round your answer to 2
significant digits.
Continue
kPa
55°F
Mostly cloudy
1
F1
F2
@
2
F3
3
F4
4
x10
X
Q Search
F5
%
5
S
F6
S
AL
A
6
F7
8
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
CO
&
7
F8
OO
>
F9
DELL
prt sc
F10
home
Submit Assi
F11
end
MEGFFEREPELHERE
A
F12
inse
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
