Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.28QAP
Related questions
Question
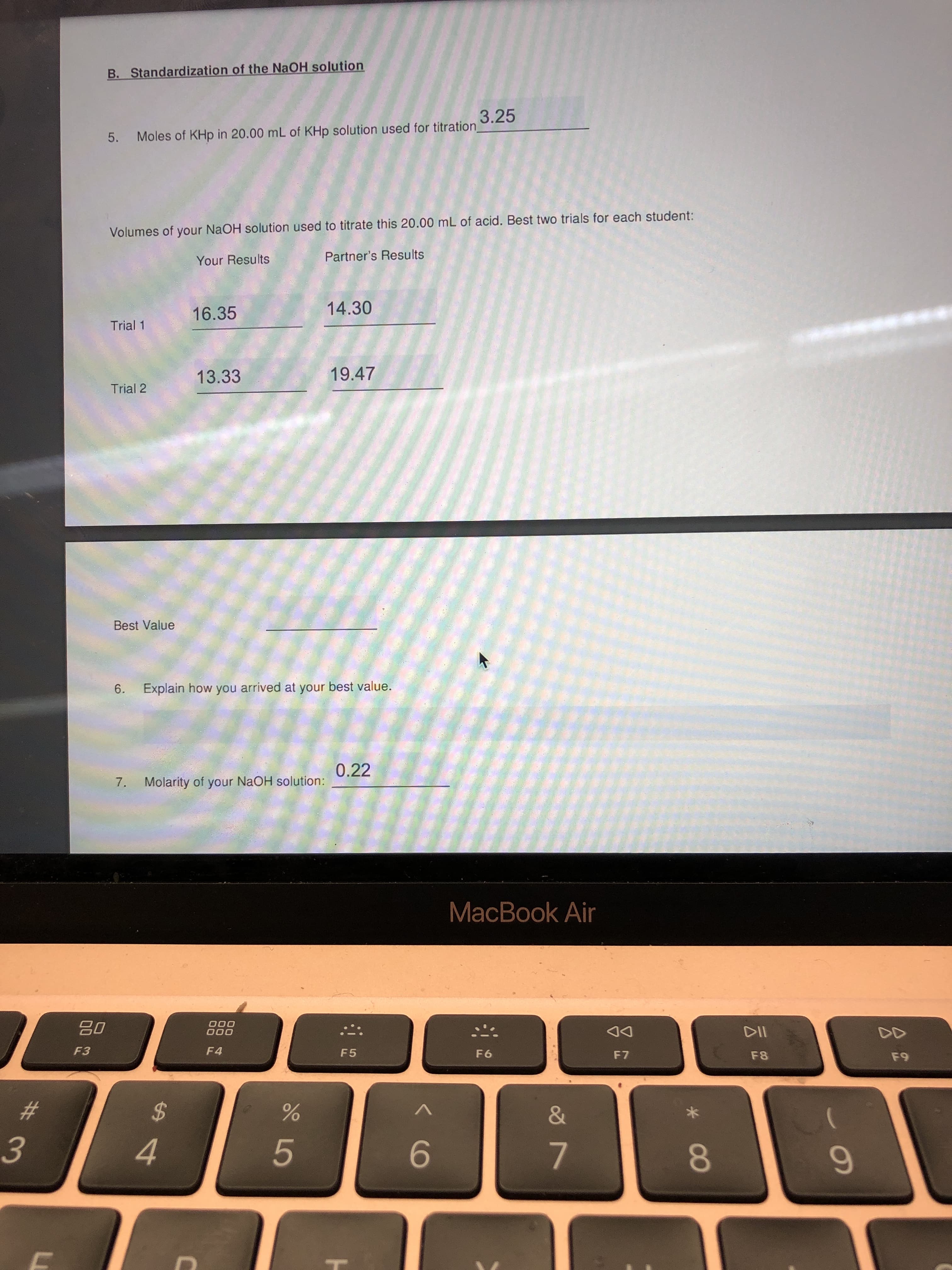
For question 5 could you find the best value and explain how you arrive to the best value please
thank you!
Transcribed Image Text:08
上
LO
%23
B. Standardization of the NaOH solution
3.25
Moles of KHp in 20.00 mL of KHp solution used for titration_
5.
Volumes of your NaOH solution used to titrate this 20.00 mL of acid. Best two trials for each student:
Your Results
Partner's Results
16.35
14.30
Trial 1
13.33
19.47
Trial 2
Best Value
6.
Explain how you arrived at your best value.
0.22
7.
Molarity of your NAOH solution:
MacBook Air
000
000
02
F4
DD
F5
F6
DA
F7
F8
%24
&
3.
4.
9-
7.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning