For the following voltaic cell (the concentrations of all metal ions are 1M) and the corresponding half reactions, Rh(ag) + 3e Rh(s) Cu*(aq)+e Cu(s) E = 0.80 V E' = 0.52 V salt bridge -Cu Rh* (49) Cu (ag) नवं In what direction do the electrons flow in the external circuit? O from the Rh anode to the Cu cathode from the Rh cathode to the Cu anode from the Cu anode to the Rh cathode from the Cu cathode to the Rh anode * Previous ASUS
For the following voltaic cell (the concentrations of all metal ions are 1M) and the corresponding half reactions, Rh(ag) + 3e Rh(s) Cu*(aq)+e Cu(s) E = 0.80 V E' = 0.52 V salt bridge -Cu Rh* (49) Cu (ag) नवं In what direction do the electrons flow in the external circuit? O from the Rh anode to the Cu cathode from the Rh cathode to the Cu anode from the Cu anode to the Rh cathode from the Cu cathode to the Rh anode * Previous ASUS
Chapter18: Introduction To Electrochemistry
Section: Chapter Questions
Problem 18.19QAP
Related questions
Question
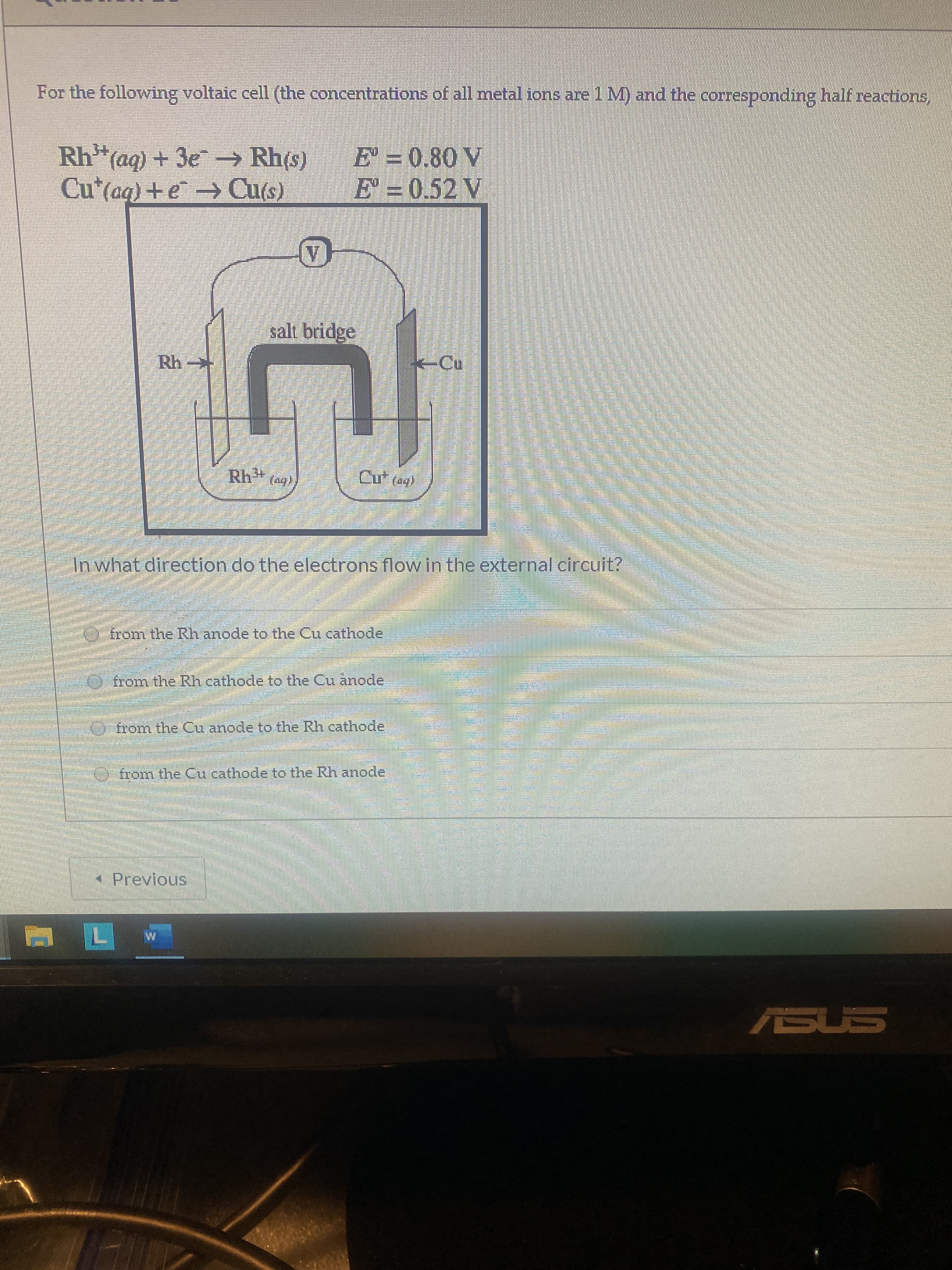
Transcribed Image Text:For the following voltaic cell (the concentrations of all metal ions are 1M) and the corresponding half reactions,
Rh(ag) + 3e Rh(s)
Cu*(aq)+e Cu(s)
E = 0.80 V
E' = 0.52 V
salt bridge
-Cu
Rh* (49)
Cu (ag)
नवं
In what direction do the electrons flow in the external circuit?
O from the Rh anode to the Cu cathode
from the Rh cathode to the Cu anode
from the Cu anode to the Rh cathode
from the Cu cathode to the Rh anode
* Previous
ASUS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning