For the NMR spectrum shown below", draw the structure of the reactant next to the spectrum and label the protons so that they match the letter labels shown on the NMR spectrum. Fill in the Chemical shift, integration and multiplicity in the table. You may want to complete sections c) and d) before assigning the aromatic ring protons. B Integration-2 There is a very broad (almost flat) signal here. Appears to be a singlet Integration-1 14 HSP-04-502 STRUCTURE: 12 10 ppm Integration-2 D Broad singlet Integration ~ 2 Proton Chemical shift Relative integration Multiplicity label (ppm) A B CD
For the NMR spectrum shown below", draw the structure of the reactant next to the spectrum and label the protons so that they match the letter labels shown on the NMR spectrum. Fill in the Chemical shift, integration and multiplicity in the table. You may want to complete sections c) and d) before assigning the aromatic ring protons. B Integration-2 There is a very broad (almost flat) signal here. Appears to be a singlet Integration-1 14 HSP-04-502 STRUCTURE: 12 10 ppm Integration-2 D Broad singlet Integration ~ 2 Proton Chemical shift Relative integration Multiplicity label (ppm) A B CD
Chapter59: Preparation Of A C-4 Or C-5 Acetate Ester
Section: Chapter Questions
Problem 6Q
Related questions
Question
Transcribed Image Text:1.
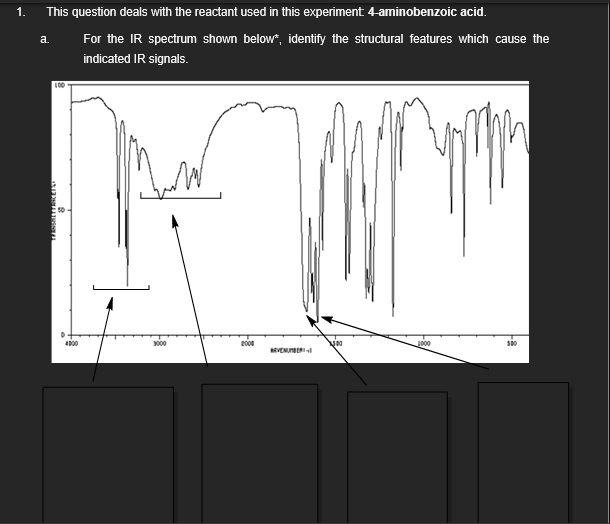
This question deals with the reactant used in this experiment 4-aminobenzoic acid.
a.
For the IR spectrum shown below*, identify the structural features which cause the
indicated IR signals.
100
4000
3000
2000
300
2000
500
ARVENUMBER
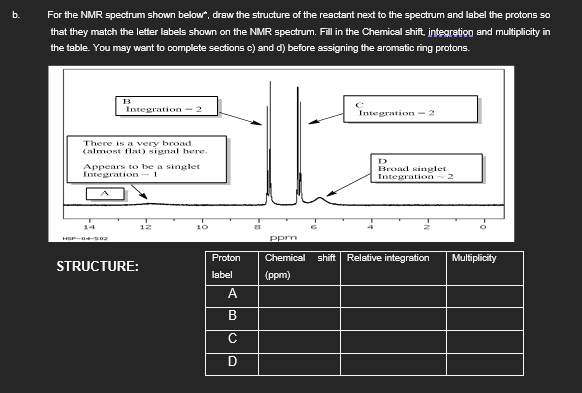
Transcribed Image Text:b.
For the NMR spectrum shown below*, draw the structure of the reactant next to the spectrum and label the protons so
that they match the letter labels shown on the NMR spectrum. Fill in the Chemical shift, integration and multiplicity in
the table. You may want to complete sections c) and d) before assigning the aromatic ring protons.
B
Integration-2
There is a very broad
(almost flat) signal here.
Appears to be a singlet
Integration - 1
14
12
10
HSP-04-502
STRUCTURE:
Integration-2
D
Broad singlet
Integration
2
ppm
Proton
Chemical
shift Relative integration
Multiplicity
label
(ppm)
с
D
ABCC
А
В
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole