For the reaction 4HCI(g) + O2(g)- →2H2O(g) + 2C1½(g) AH° = -114.4 kJ and AS° = -128.9 J/K The standard free energy change for the reaction of 1.62 moles of HCl(g) at 332 K, 1 atm would be kJ. This reaction is (reactant, product) favored under standard conditions at 332 K. Assume that AH° and AS° are independent of temperature.
For the reaction 4HCI(g) + O2(g)- →2H2O(g) + 2C1½(g) AH° = -114.4 kJ and AS° = -128.9 J/K The standard free energy change for the reaction of 1.62 moles of HCl(g) at 332 K, 1 atm would be kJ. This reaction is (reactant, product) favored under standard conditions at 332 K. Assume that AH° and AS° are independent of temperature.
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 70E: Hydrogen sulfide can be removed from natural gas by the reaction 2H2S(g)+SO2(g)3S(s)+2H2O(g)...
Related questions
Question
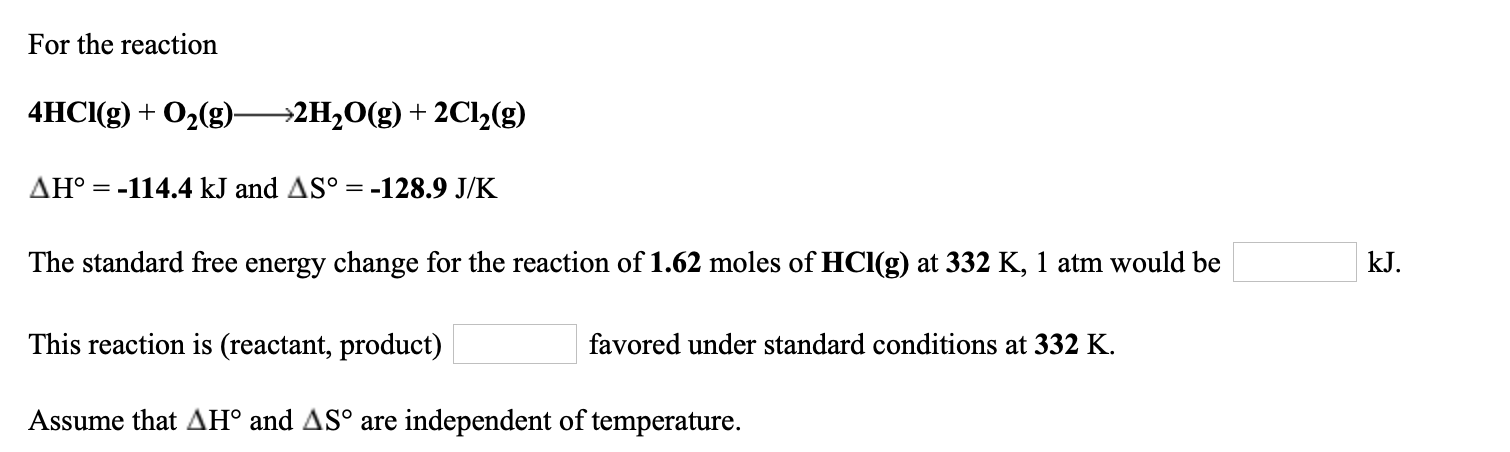
Transcribed Image Text:For the reaction
4HCI(g) + O2(g)-
→2H2O(g) + 2C1½(g)
AH° = -114.4 kJ and AS° = -128.9 J/K
The standard free energy change for the reaction of 1.62 moles of HCl(g) at 332 K, 1 atm would be
kJ.
This reaction is (reactant, product)
favored under standard conditions at 332 K.
Assume that AH° and AS° are independent of temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co