Four closed tanks, A, B, C, and D, each contain an ideal gas. The table gives the absolute pressure and volume of the gas in each tank. In each case, there is 0.13 mol of gas. Using this number and the data in the table, compute the temperature of the gas in each tank. B Absolute pressure (Pa) Volume (m) 25.0 30.0 20.0 2.0 4.0 5.0 5.0 75
Four closed tanks, A, B, C, and D, each contain an ideal gas. The table gives the absolute pressure and volume of the gas in each tank. In each case, there is 0.13 mol of gas. Using this number and the data in the table, compute the temperature of the gas in each tank. B Absolute pressure (Pa) Volume (m) 25.0 30.0 20.0 2.0 4.0 5.0 5.0 75
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter13: Temperature, Kinetic Theory, And The Gas Laws
Section: Chapter Questions
Problem 37PE: (a) What is me gauge pressure in a 25.0C car tire containing 3.60 mol of gas in a 30.0 L volume? (b)...
Related questions
Question
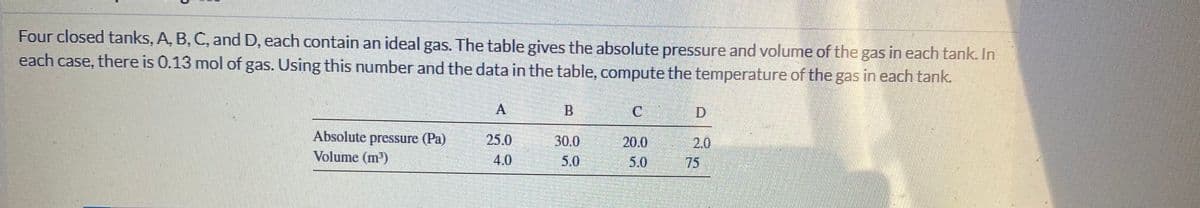
Transcribed Image Text:Four closed tanks, A, B, C, and D, each contain an ideal gas. The table gives the absolute pressure and volume of the gas in each tank. In
each case, there is 0.13 mol of gas. Using this number and the data in the table, compute the temperature of the gas in each tank.
A
B.
Absolute pressure (Pa)
Volume (m³)
25.0
30.0
20.0
2.0
75
4.0
5.0
5.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning