Freezing Point of pure TBA (experimentally determined) = 22.90 °C. 8.09 (C)(kg solvent) mol solute Molal Freezing Point Depression Constant 0'00" (0.00) 37.00 0'15" (0.25) 31.40 0'30" (0.50) 27.50 0'45" (0.75) 23.80 1'00" (1.00) 21.00 1'15" (1.25) 18.80 1'30" (1.50) 16.60 Data-Freezing Point with 1" addition of Solute Time min'sec" Temperature Time min'sec" Temperature (decimal) (°C) (decimal) (°C) Freezing Point Data A Time (min) Temp ( 0.00 0.25 0.50 0.75 1.00 1.25 1.50 16.60 1.75 2.00 2.50 3.00 3.50 4.00 4.50 5.00 5.50 6.00 37.00 Freesing Point Data B 31.40 Time (min) Temp (C) 27.50 0.50 23.80 21.00 18.80 ml -13.271 61 35.111 Intersection Temp 0.75 1.00 1.25 1.50 1.75 15.50 2.00 15.00 2.50 14.50 3.00 3.50 4.00 4.50 13.70 13.00 12.20 11.60 5.00 11.00 5.50 10.40 6.00 9.80 m2 -1.35 62 17.75 15.79 °C 1'45" (1.75) 15.50 2'00" (2.00) 15.00 2'30" (2.50) 14.50 3'00" (3.00) 13.70 3'30" (3.50) 13.00 4'00" (4.00) 12.20 4'30" (4.50) 11.60 40.00 35.00 30.00 Brookhaven College Chemistry - v6a 25.00 20.00 15.00 10.00 5.00 ■ 0.00 TBA ; k, = 1.00 y-13271 35.411 2.00 Freezing Point Determination Temperature (°C) vs Time (min) 1.00 Time min'sec" Temperature (decimal) (°C) 5'00" (5.00) 11.00 5'30" (5.50) 10.40 6'00" (6.00) 9.80 4.00 Time (min) Molal Freezing Point Depression Constant, k, for TBA= Page 1 of 12 ya-1.3457x + 17,75 a) What is the freezing point of the solution? b) What is the molar mass of the unknown? 2. Construct a Data Table for the collection of your experimental data. Hint: Read your experiment carefully to determine what data you will need to collect and organize it so that it will be easy to use and follow throughout your experiment. Pre-Lab Question I may give you some ideas on how to construct the data sheet. 8.09 5.00 The one constant that you will need to include is the Molal Freezing Point Depression Constant for TBA. 6.00 1/7/2020 7.00 (C)(kg solvent) mol solute X2068
Freezing Point of pure TBA (experimentally determined) = 22.90 °C. 8.09 (C)(kg solvent) mol solute Molal Freezing Point Depression Constant 0'00" (0.00) 37.00 0'15" (0.25) 31.40 0'30" (0.50) 27.50 0'45" (0.75) 23.80 1'00" (1.00) 21.00 1'15" (1.25) 18.80 1'30" (1.50) 16.60 Data-Freezing Point with 1" addition of Solute Time min'sec" Temperature Time min'sec" Temperature (decimal) (°C) (decimal) (°C) Freezing Point Data A Time (min) Temp ( 0.00 0.25 0.50 0.75 1.00 1.25 1.50 16.60 1.75 2.00 2.50 3.00 3.50 4.00 4.50 5.00 5.50 6.00 37.00 Freesing Point Data B 31.40 Time (min) Temp (C) 27.50 0.50 23.80 21.00 18.80 ml -13.271 61 35.111 Intersection Temp 0.75 1.00 1.25 1.50 1.75 15.50 2.00 15.00 2.50 14.50 3.00 3.50 4.00 4.50 13.70 13.00 12.20 11.60 5.00 11.00 5.50 10.40 6.00 9.80 m2 -1.35 62 17.75 15.79 °C 1'45" (1.75) 15.50 2'00" (2.00) 15.00 2'30" (2.50) 14.50 3'00" (3.00) 13.70 3'30" (3.50) 13.00 4'00" (4.00) 12.20 4'30" (4.50) 11.60 40.00 35.00 30.00 Brookhaven College Chemistry - v6a 25.00 20.00 15.00 10.00 5.00 ■ 0.00 TBA ; k, = 1.00 y-13271 35.411 2.00 Freezing Point Determination Temperature (°C) vs Time (min) 1.00 Time min'sec" Temperature (decimal) (°C) 5'00" (5.00) 11.00 5'30" (5.50) 10.40 6'00" (6.00) 9.80 4.00 Time (min) Molal Freezing Point Depression Constant, k, for TBA= Page 1 of 12 ya-1.3457x + 17,75 a) What is the freezing point of the solution? b) What is the molar mass of the unknown? 2. Construct a Data Table for the collection of your experimental data. Hint: Read your experiment carefully to determine what data you will need to collect and organize it so that it will be easy to use and follow throughout your experiment. Pre-Lab Question I may give you some ideas on how to construct the data sheet. 8.09 5.00 The one constant that you will need to include is the Molal Freezing Point Depression Constant for TBA. 6.00 1/7/2020 7.00 (C)(kg solvent) mol solute X2068
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.12QAP
Related questions
Question
I need help with Q b please
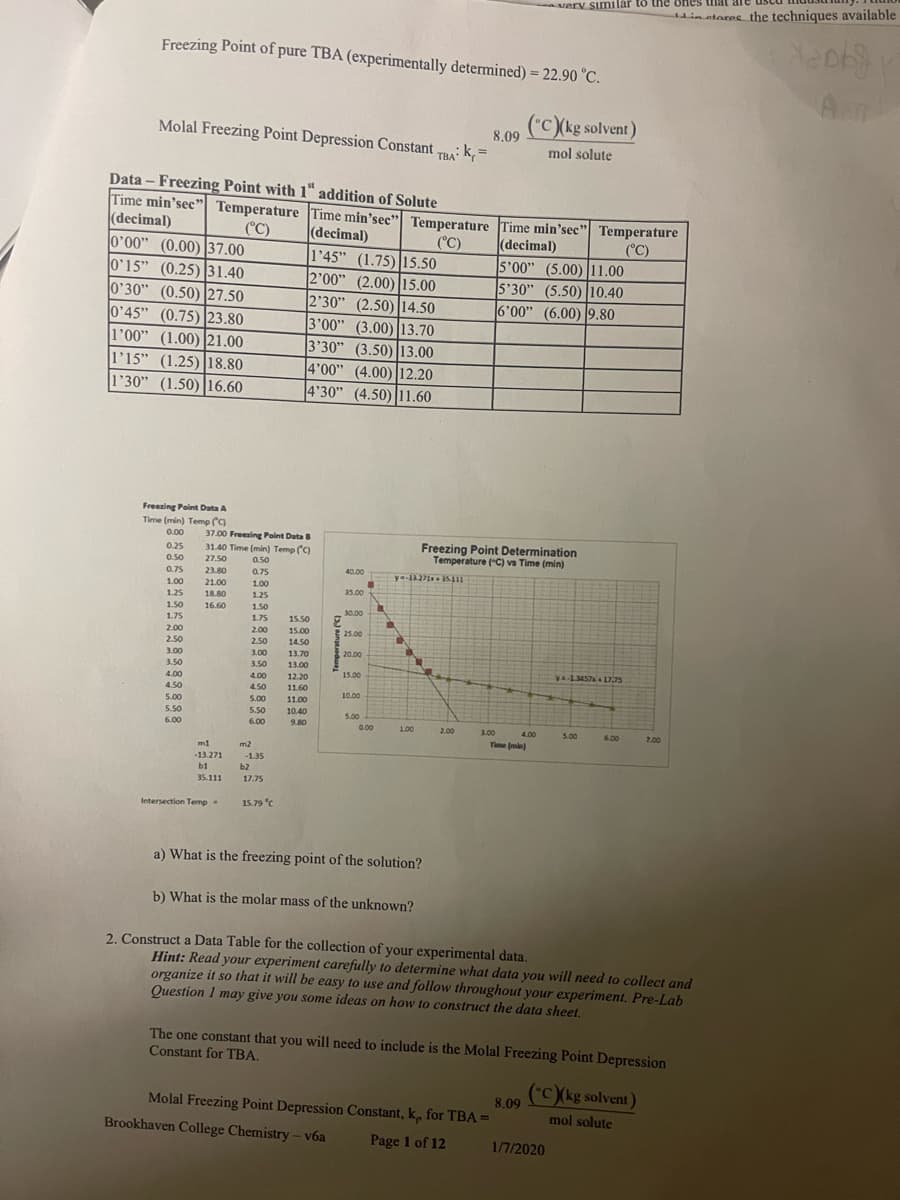
Transcribed Image Text:Freezing Point of pure TBA (experimentally determined) = 22.90 °C.
("C)(kg solvent)
mol solute
8.09
Molal Freezing Point Depression Constant TBA: k, =
Data- Freezing Point with 1" addition of Solute
Time min'sec" Temperature Time min'sec" Temperature
(decimal)
(°C) (decimal)
(°C)
0'00" (0.00) 37.00
0'15" (0.25) 31.40
0'30" (0.50) 27.50
0'45" (0.75) 23.80
1'00" (1.00) 21.00
1'15" (1.25) 18.80
1'30" (1.50) 16.60
Freezing Point Data A
Time (min) Temp (C)
0.00
1.50
1.75
37.00 Freezing Point Data B
31.40 Time (min) Temp (C)
0.25
0.50
27.50
0.50
0.75 23.80
0.75
1.00
21.00
1.00
1.25
18.80
1.25
16.60
1.50
1.75
2.00
2.00
2.50
3.00
3.50
4.00
4.50
5.00
5.50
6.00
m1
-13.271
b1
35.111
Intersection Temp
15.50
15.00
2.50
14.50
3.00 13.70
3.50
13.00
4.00
12.20
4.50
11.60
5.00
5.50
6.00
m2
-1.35
b2
17.75
15.79 °C
1'45" (1.75) 15.50
2'00" (2.00) 15.00
2'30" (2.50) 14.50
3'00" (3.00) 13.70
3'30" (3.50) 13.00
4'00" (4.00) 12.20
4'30" (4.50) 11.60
11.00
10.40
9.80
40.00
35.00
30.00
25.00
20.00
15.00
10.00
5.00
0.00
ya-1327135.111
1.00
a) What is the freezing point of the solution?
Freezing Point Determination
Temperature (°C) vs Time (min)
2.00
3.00
Time min'sec" Temperature
(decimal)
(°C)
5'00" (5.00) 11.00
5'30" (5.50) 10.40
6'00" (6.00) 9.80
very similar to the ones that
4.00
Time (min)
Molal Freezing Point Depression Constant, k, for TBA=
Brookhaven College Chemistry - v6a
Page 1 of 12
8,09
ya-1.3457x + 17,75
5.00
1/7/2020
6.00
b) What is the molar mass of the unknown?
2. Construct a Data Table for the collection of your experimental data.
Hint: Read your experiment carefully to determine what data you will need to collect and
organize it so that it will be easy to use and follow throughout your experiment. Pre-Lab
Question I may give you some ideas on how to construct the data sheet.
The one constant that you will need to include is the Molal Freezing Point Depression
Constant for TBA.
7.00
(C)(kg solvent)
mol solute
intores the techniques available
206
AST

Transcribed Image Text:PRE-LAB QUESTIONS
1. The following experimental data were collected and graphed:
Mass of pure TBA used = 15.348 g
1.081 g
Mass of FIRST Sample in TBA=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning