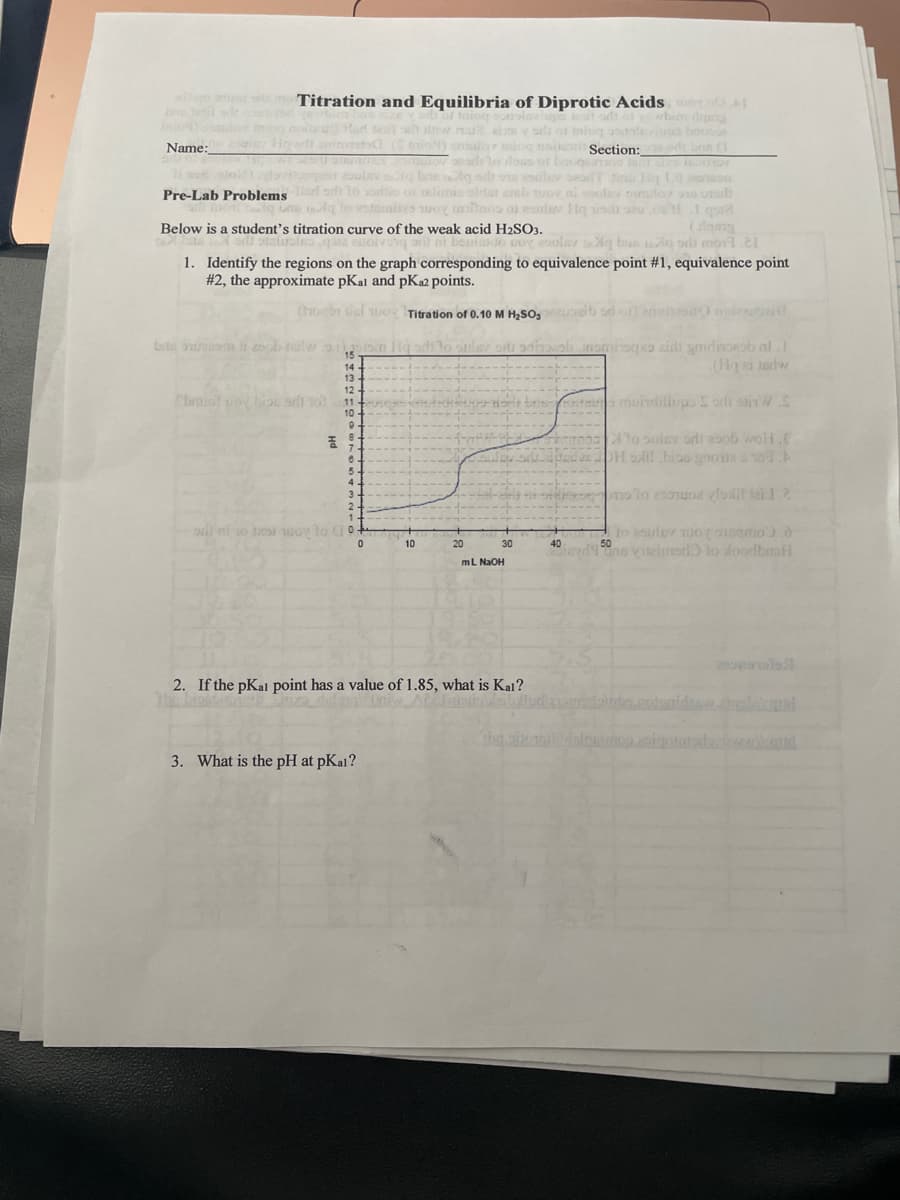
gb nge men by aa to cougn 2om caoe Below is a student's titration curve of the weak acid H2SO3. DoY esolar g bus oli mot. 1. Identify the regions on the graph corresponding to equivalence point #1, equivalence point #2, the approximate pKal and pKa2 points. ( n dul woy Titration of 0.10o M H,SO, ib brte ns t d noniroqo aidi gmidincorob al.1 15 14 13 12 br adi 10 muindilhups o olinW.S 11 10 To sulsv ordi esob wol.E OH lil biao gnone 0. 7. 4. 3. 2oulov uo amo.d 50 10 20 30 40 Vileimad to dondbmfl mL NaOH 2. If the pKal point has a value of 1.85, what is Kal? ni 3. What is the pH at pKal? Hd
gb nge men by aa to cougn 2om caoe Below is a student's titration curve of the weak acid H2SO3. DoY esolar g bus oli mot. 1. Identify the regions on the graph corresponding to equivalence point #1, equivalence point #2, the approximate pKal and pKa2 points. ( n dul woy Titration of 0.10o M H,SO, ib brte ns t d noniroqo aidi gmidincorob al.1 15 14 13 12 br adi 10 muindilhups o olinW.S 11 10 To sulsv ordi esob wol.E OH lil biao gnone 0. 7. 4. 3. 2oulov uo amo.d 50 10 20 30 40 Vileimad to dondbmfl mL NaOH 2. If the pKal point has a value of 1.85, what is Kal? ni 3. What is the pH at pKal? Hd
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.17QAP
Related questions
Question
I need help completing this page
Transcribed Image Text:mier moTitration and Equilibria of Diprotic Acids H
bin dg
u ais y ols
omalor niog nolesi Section: brue (1
lo doss
Name:
bacqan
Pre-Lab Problems
eldat steb uov
Below is a student's titration curve of the weak acid H2SO3.
Doy solav qg bua q oili mo1.21
8 ni ben
1. Identify the regions on the graph corresponding to equivalence point #1, equivalence point
#2, the approximate pKal and pK2 points.
Con dul uo Titration of 0.10 M H2SO,
brte t 2o
15
14
13.
Cbr soy biou sdi 10
12 -
11-
10 -
ntao muindilhp orls otin W.C
To sulav ordi esob woH.E
H SAil bioo gnone a109.A
o lo 2 oz vlolit tai 12
10
30
50
40
iay19ne vileimad to loodbmfl
mL NaOH
2. If the pKal point has a value of 1.85, what is Kal?
opoigotuda d
3. What is the pH at pKal?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning